Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis
- PMID: 20154217
- PMCID: PMC2854434
- DOI: 10.1182/blood-2009-04-218842
Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis
Abstract
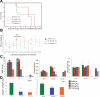
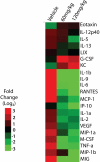
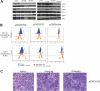
The discovery of JAK2 and MPL mutations in patients with myeloproliferative neoplasms (MPNs) provided important insight into the genetic basis of these disorders and led to the development of JAK2 kinase inhibitors for MPN therapy. Although recent studies have shown that JAK2 kinase inhibitors demonstrate efficacy in a JAK2V617F murine bone marrow transplantation model, the effects of JAK2 inhibitors on MPLW515L-mediated myeloproliferation have not been investigated. In this report, we describe the in vitro and in vivo effects of INCB16562, a small-molecule JAK2 inhibitor. INCB16562 inhibited proliferation and signaling in cell lines transformed by JAK2 and MPL mutations. Compared with vehicle treatment, INCB16562 treatment improved survival, normalized white blood cell counts and platelet counts, and markedly reduced extramedullary hematopoeisis and bone marrow fibrosis. We observed inhibition of STAT3 and STAT5 phosphorylation in vivo consistent with potent inhibition of JAK-STAT signaling. These data suggest JAK2 inhibitor therapy may be of value in the treatment of JAK2V617F-negative MPNs. However, we did not observe a decrease in the size of the malignant clone in the bone marrow of treated mice at the end of therapy, which suggests that JAK2 inhibitor therapy, by itself, was not curative in this MPN model.
Figures

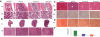
Similar articles
-
MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia.PLoS Med. 2006 Jul;3(7):e270. doi: 10.1371/journal.pmed.0030270. PLoS Med. 2006. PMID: 16834459 Free PMC article.
-
Targeting compensatory MEK/ERK activation increases JAK inhibitor efficacy in myeloproliferative neoplasms.J Clin Invest. 2019 Mar 4;129(4):1596-1611. doi: 10.1172/JCI98785. eCollection 2019 Mar 4. J Clin Invest. 2019. PMID: 30730307 Free PMC article.
-
EXEL-8232, a small-molecule JAK2 inhibitor, effectively treats thrombocytosis and extramedullary hematopoiesis in a murine model of myeloproliferative neoplasm induced by MPLW515L.Leukemia. 2012 Apr;26(4):720-7. doi: 10.1038/leu.2011.261. Epub 2011 Oct 18. Leukemia. 2012. PMID: 22005786
-
The Development and Use of Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms.Hematol Oncol Clin North Am. 2017 Aug;31(4):613-626. doi: 10.1016/j.hoc.2017.04.002. Epub 2017 May 17. Hematol Oncol Clin North Am. 2017. PMID: 28673391 Review.
-
Narrative review: Thrombocytosis, polycythemia vera, and JAK2 mutations: The phenotypic mimicry of chronic myeloproliferation.Ann Intern Med. 2010 Mar 2;152(5):300-6. doi: 10.7326/0003-4819-152-5-201003020-00008. Ann Intern Med. 2010. PMID: 20194236 Review.
Cited by
-
Unraveling the genetic underpinnings of myeloproliferative neoplasms and understanding their effect on disease course and response to therapy: proceedings from the 6th International Post-ASH Symposium.Am J Hematol. 2012 May;87(5):562-8. doi: 10.1002/ajh.23169. Epub 2012 Mar 28. Am J Hematol. 2012. PMID: 22460584 Free PMC article.
-
Signal transduction in the chronic leukemias: implications for targeted therapies.Curr Hematol Malig Rep. 2013 Mar;8(1):71-80. doi: 10.1007/s11899-012-0150-1. Curr Hematol Malig Rep. 2013. PMID: 23307472 Free PMC article. Review.
-
JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response.Cancer Discov. 2015 Mar;5(3):316-31. doi: 10.1158/2159-8290.CD-14-0736. Epub 2015 Jan 8. Cancer Discov. 2015. PMID: 25572172 Free PMC article.
-
Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy.Nature. 2012 Sep 6;489(7414):155-9. doi: 10.1038/nature11303. Nature. 2012. PMID: 22820254 Free PMC article.
-
MPL W515L expression induces TGFβ secretion and leads to an increase in chemokinesis via phosphorylation of THOC5.Oncotarget. 2016 Mar 8;7(10):10739-55. doi: 10.18632/oncotarget.7639. Oncotarget. 2016. PMID: 26919114 Free PMC article.
References
-
- Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355(23):2452–2466. - PubMed
-
- Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110(4):1092–1097. - PubMed
-
- Ma X, Vanasse G, Cartmel B, Wang Y, Selinger HA. Prevalence of polycythemia vera and essential thrombocythemia. Am J Hematol. 2008;83(5):359–362. - PubMed
-
- Tefferi A. Essential thrombocythemia, polycythemia vera, and myelofibrosis: current management and the prospect of targeted therapy. Am J Hematol. 2008;83(6):491–497. - PubMed
-
- Deeg HJ, Gooley TA, Flowers ME, et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Blood. 2003;102(12):3912–3918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

