Effects of tempol and redox-cycling nitroxides in models of oxidative stress
- PMID: 20153367
- PMCID: PMC2854323
- DOI: 10.1016/j.pharmthera.2010.01.003
Effects of tempol and redox-cycling nitroxides in models of oxidative stress
Abstract
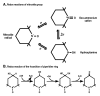
Tempol is a redox-cycling nitroxide that promotes the metabolism of many reactive oxygen species (ROS) and improves nitric oxide bioavailability. It has been studied extensively in animal models of oxidative stress. Tempol has been shown to preserve mitochondria against oxidative damage and improve tissue oxygenation. Tempol improved insulin responsiveness in models of diabetes mellitus and improved the dyslipidemia, reduced the weight gain and prevented diastolic dysfunction and heart failure in fat-fed models of the metabolic syndrome. Tempol protected many organs, including the heart and brain, from ischemia/reperfusion damage. Tempol prevented podocyte damage, glomerulosclerosis, proteinuria and progressive loss of renal function in models of salt and mineralocorticosteroid excess. It reduced brain or spinal cord damage after ischemia or trauma and exerted a spinal analgesic action. Tempol improved survival in several models of shock. It protected normal cells from radiation while maintaining radiation sensitivity of tumor cells. Its paradoxical pro-oxidant action in tumor cells accounted for a reduction in spontaneous tumor formation. Tempol was effective in some models of neurodegeneration. Thus, tempol has been effective in preventing several of the adverse consequences of oxidative stress and inflammation that underlie radiation damage and many of the diseases associated with aging. Indeed, tempol given from birth prolonged the life span of normal mice. However, presently tempol has been used only in human subjects as a topical agent to prevent radiation-induced alopecia.
Copyright 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice.PLoS One. 2014 Oct 1;9(10):e108889. doi: 10.1371/journal.pone.0108889. eCollection 2014. PLoS One. 2014. PMID: 25271439 Free PMC article.
-
Impact of polynitroxylated albumin (PNA) and tempol on ischemia/reperfusion injury: intravital microscopic study in the dorsal skinfold chamber of the Syrian golden hamster.Shock. 2000 Aug;14(2):163-8. doi: 10.1097/00024382-200014020-00015. Shock. 2000. PMID: 10947161
-
Effects of tempol, a free radical scavenger, on long-term hyperdynamic porcine bacteremia.Crit Care Med. 2005 May;33(5):1057-63. doi: 10.1097/01.ccm.0000162927.94753.63. Crit Care Med. 2005. PMID: 15891336
-
The Cellular and Organismal Effects of Nitroxides and Nitroxide-Containing Nanoparticles.Int J Mol Sci. 2024 Jan 24;25(3):1446. doi: 10.3390/ijms25031446. Int J Mol Sci. 2024. PMID: 38338725 Free PMC article. Review.
-
[Nitroxides as antioxidants - possibilities of their application in chemoprevention and radioprotection].Postepy Hig Med Dosw (Online). 2011 Feb 2;65:46-54. doi: 10.5604/17322693.932256. Postepy Hig Med Dosw (Online). 2011. PMID: 21357994 Review. Polish.
Cited by
-
Thromboxane prostanoid receptors enhance contractions, endothelin-1, and oxidative stress in microvessels from mice with chronic kidney disease.Hypertension. 2015 May;65(5):1055-63. doi: 10.1161/HYPERTENSIONAHA.115.05244. Epub 2015 Mar 2. Hypertension. 2015. PMID: 25733239 Free PMC article.
-
Cytochrome P450 1B1 Contributes to the Development of Angiotensin II-Induced Aortic Aneurysm in Male Apoe(-/-) Mice.Am J Pathol. 2016 Aug;186(8):2204-2219. doi: 10.1016/j.ajpath.2016.04.005. Epub 2016 Jun 11. Am J Pathol. 2016. PMID: 27301358 Free PMC article.
-
High Salt Enhances Reactive Oxygen Species and Angiotensin II Contractions of Glomerular Afferent Arterioles From Mice With Reduced Renal Mass.Hypertension. 2018 Nov;72(5):1208-1216. doi: 10.1161/HYPERTENSIONAHA.118.11354. Hypertension. 2018. PMID: 30354808 Free PMC article.
-
Effects of the hydroalcoholic extract of Rosa damascena on learning and memory in male rats consuming a high-fat diet.Pharm Biol. 2017 Dec;55(1):2065-2073. doi: 10.1080/13880209.2017.1362010. Pharm Biol. 2017. PMID: 28832226 Free PMC article.
-
Photocatalytic Nanowires-Based Air Filter: Towards Reusable Protective Masks.Adv Funct Mater. 2020 Oct 1;30(40):2004615. doi: 10.1002/adfm.202004615. Epub 2020 Aug 7. Adv Funct Mater. 2020. PMID: 32837497 Free PMC article.
References
-
- Adeagbo AS, Joshua IG, Falkner C, Matheson PJ. Tempol, an antioxidant, restores endothelium-derived hyperpolarizing factor-mediated vasodilation during hypertension. Eur J Pharmacol. 2003;481:91–100. - PubMed
-
- Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, et al. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol. 2003;285:H1015–1022. - PubMed
-
- Akiyama N, Umeda IO, Sogo S, Nishigori H, Tsujimoto M, Natori S. 5-S-GAD, a novel radical scavenging compound, prevents lens opacity development. Free Radic Biol Med. 2009;46:511–519. - PubMed
-
- Alpert E, Altman H, Totary H, Gruzman A, Barnea D, Barash V, et al. 4-Hydroxy tempol-induced impairment of mitochondrial function and augmentation of glucose transport in vascular endothelial and smooth muscle cells. Biochem Pharmacol. 2004;67:1985–1995. - PubMed
-
- Ando K, Fujita T. Metabolic syndrome and oxidative stress. Free Radic Biol Med. 2009;47:213–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

