Serum cellular apoptosis susceptibility protein is a potential prognostic marker for metastatic colorectal cancer
- PMID: 20150437
- PMCID: PMC2843454
- DOI: 10.2353/ajpath.2010.090467
Serum cellular apoptosis susceptibility protein is a potential prognostic marker for metastatic colorectal cancer
Abstract
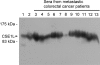
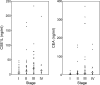
Colorectal cancer has high rates of recurrence and metastasis. Many patients with similar histopathological features show significantly different clinical outcomes, and these differences are primarily related to metastases undetected by current diagnostic methods. There is no useful serological marker for metastatic disease. We investigated the cellular apoptosis susceptibility (CSE1L/CAS) protein in comparison with carcinoembryonic antigen (CEA) as a marker for metastatic colorectal cancer. Using serum from 103 patients with stage I, II, III, and IV disease, CSE1L was detected in 36.0% (9 of 25), 57.7% (15 of 26), 71.4% (30 of 42), and 88.9% (8 of 9) of patients, respectively; a pathological CEA level was found in 16.0% (4 of 25), 42.3% (11 of 26), 47.6% (20 of 42), and 77.8% (7 of 9) of patients, respectively; a combined CSE1L/CEA assay was detected in 48.0% (12 of 25), 65.4% (17 of 26), 88.1% (37 of 42), and 100% (9 of 9) of patients, respectively. Lymphatic metastasis is an important predictor of poor prognosis and crucial for determination of therapeutic strategy. Serum CSE1L was detected in 74.5% (38 of 51) of patients with lymph node metastasis, whereas a pathological CEA level was found in only 52.9% (27 of 51) of the same patients (P < 0.001); the combined CSE1L/CEA assay increased sensitivity to 90.2% (46 of 51). Animal experiments showed CSE1L reduction in B16-F10 melanoma cells correlated with decreased metastasis to the colorectal tract in C57BL/6 mice. These results indicate that assay of serum CSE1L may facilitate diagnosis of colorectal cancer lymphatic metastases; furthermore, CSE1L is a possible therapeutic target.
Figures



Similar articles
-
Correlations between cytoplasmic CSE1L in neoplastic colorectal glands and depth of tumor penetration and cancer stage.J Transl Med. 2013 Jan 31;11:29. doi: 10.1186/1479-5876-11-29. J Transl Med. 2013. PMID: 23369209 Free PMC article.
-
Cellular apoptosis susceptibility (chromosome segregation 1-like, CSE1L) gene is a key regulator of apoptosis, migration and invasion in colorectal cancer.J Pathol. 2012 Dec;228(4):471-81. doi: 10.1002/path.4031. Epub 2012 Aug 20. J Pathol. 2012. PMID: 22450763
-
CSE1L modulates Ras-induced cancer cell invasion: correlation of K-Ras mutation and CSE1L expression in colorectal cancer progression.Am J Surg. 2013 Sep;206(3):418-27. doi: 10.1016/j.amjsurg.2012.11.021. Epub 2013 Jun 24. Am J Surg. 2013. PMID: 23806821
-
Cellular apoptosis susceptibility (CSE1L/CAS) protein in cancer metastasis and chemotherapeutic drug-induced apoptosis.J Exp Clin Cancer Res. 2010 Aug 11;29(1):110. doi: 10.1186/1756-9966-29-110. J Exp Clin Cancer Res. 2010. PMID: 20701792 Free PMC article. Review.
-
CAS (CSE1L) signaling pathway in tumor progression and its potential as a biomarker and target for targeted therapy.Tumour Biol. 2016 Oct;37(10):13077-13090. doi: 10.1007/s13277-016-5301-x. Epub 2016 Sep 5. Tumour Biol. 2016. PMID: 27596143 Review.
Cited by
-
CSE1L, a novel microvesicle membrane protein, mediates Ras-triggered microvesicle generation and metastasis of tumor cells.Mol Med. 2012 Dec 6;18(1):1269-80. doi: 10.2119/molmed.2012.00205. Mol Med. 2012. PMID: 22952058 Free PMC article.
-
A comprehensive study of immunology repertoires in both preoperative stage and postoperative stage in patients with colorectal cancer.Mol Genet Genomic Med. 2019 Mar;7(3):e504. doi: 10.1002/mgg3.504. Epub 2019 Jan 9. Mol Genet Genomic Med. 2019. PMID: 30628178 Free PMC article.
-
Correlations between cytoplasmic CSE1L in neoplastic colorectal glands and depth of tumor penetration and cancer stage.J Transl Med. 2013 Jan 31;11:29. doi: 10.1186/1479-5876-11-29. J Transl Med. 2013. PMID: 23369209 Free PMC article.
-
CSE1L, DIDO1 and RBM39 in colorectal adenoma to carcinoma progression.Cell Oncol (Dordr). 2012 Aug;35(4):293-300. doi: 10.1007/s13402-012-0088-2. Epub 2012 Jun 19. Cell Oncol (Dordr). 2012. PMID: 22711543
-
Early detection of colorectal cancer: from conventional methods to novel biomarkers.J Cancer Res Clin Oncol. 2016 Feb;142(2):341-51. doi: 10.1007/s00432-015-1928-z. Epub 2015 Feb 17. J Cancer Res Clin Oncol. 2016. PMID: 25687380 Review.
References
-
- Lurje G, Zhang W, Lenz HJ. Molecular prognostic markers in locally advanced colon cancer. Clin Colorectal Cancer. 2007;6:683–690. - PubMed
-
- Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J, Labianca R, Chen W, Cha SS, Heldebrant MP, Goldberg RM. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. - PubMed
-
- Carlson MR. Previstage GCC colorectal cancer staging test: a new molecular test to identify lymph node metastases and provide more accurate information about the stage of patients with colorectal cancer. Mol Diagn Ther. 2009;13:11–14. - PubMed
-
- Losi L, Ponti G, Gregorio CD, Marino M, Rossi G, Pedroni M, Benatti P, Roncucci L, de Leon MP. Prognostic significance of histological features and biological parameters in stage I (pT1 and pT2) colorectal adenocarcinoma. Pathol Res Pract. 2006;202:663–670. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

