ATP dependent chromatin remodeling enzymes in embryonic stem cells
- PMID: 20148317
- PMCID: PMC2862992
- DOI: 10.1007/s12015-010-9120-y
ATP dependent chromatin remodeling enzymes in embryonic stem cells
Abstract
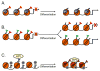
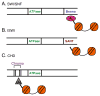
Embryonic stem (ES) cells are pluripotent cells that can self renew or be induced to differentiate into multiple cell lineages, and thus have the potential to be utilized in regenerative medicine. Key pluripotency specific factors (Oct 4/Sox2/Nanog/Klf4) maintain the pluripotent state by activating expression of pluripotency specific genes and by inhibiting the expression of developmental regulators. Pluripotent ES cells are distinguished from differentiated cells by a specialized chromatin state that is required to epigenetically regulate the ES cell phenotype. Recent studies show that in addition to pluripotency specific factors, chromatin remodeling enzymes play an important role in regulating ES cell chromatin and the capacity to self-renew and to differentiate. Here we review recent studies that delineate the role of ATP dependent chromatin remodeling enzymes in regulating ES cell chromatin structure.
Figures
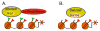
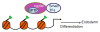
Similar articles
-
Chromatin remodeling in embryonic stem cells: regulating the balance between pluripotency and differentiation.J Cell Physiol. 2009 Apr;219(1):1-7. doi: 10.1002/jcp.21654. J Cell Physiol. 2009. PMID: 19097034 Review.
-
Control of embryonic stem cell identity by nucleosome remodeling enzymes.Curr Opin Genet Dev. 2010 Oct;20(5):500-4. doi: 10.1016/j.gde.2010.08.001. Epub 2010 Aug 25. Curr Opin Genet Dev. 2010. PMID: 20800472 Free PMC article. Review.
-
ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a.Proc Natl Acad Sci U S A. 2008 May 6;105(18):6656-61. doi: 10.1073/pnas.0801802105. Epub 2008 Apr 30. Proc Natl Acad Sci U S A. 2008. PMID: 18448678 Free PMC article.
-
BAF250B-associated SWI/SNF chromatin-remodeling complex is required to maintain undifferentiated mouse embryonic stem cells.Stem Cells. 2008 May;26(5):1155-65. doi: 10.1634/stemcells.2007-0846. Epub 2008 Mar 6. Stem Cells. 2008. PMID: 18323406 Free PMC article.
-
Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells.Genes Dev. 2007 Oct 15;21(20):2545-57. doi: 10.1101/gad.1588207. Genes Dev. 2007. PMID: 17938240 Free PMC article.
Cited by
-
SEL1L SNP rs12435998, a predictor of glioblastoma survival and response to radio-chemotherapy.Oncotarget. 2015 May 20;6(14):12452-67. doi: 10.18632/oncotarget.3611. Oncotarget. 2015. PMID: 25948789 Free PMC article.
-
The CHD family chromatin remodeling enzyme, Kismet, promotes both clathrin-mediated and activity-dependent bulk endocytosis.PLoS One. 2024 Mar 21;19(3):e0300255. doi: 10.1371/journal.pone.0300255. eCollection 2024. PLoS One. 2024. PMID: 38512854 Free PMC article.
-
Epigenetic landscape of pluripotent stem cells.Antioxid Redox Signal. 2012 Jul 15;17(2):205-23. doi: 10.1089/ars.2011.4375. Epub 2012 Jan 11. Antioxid Redox Signal. 2012. PMID: 22044221 Free PMC article. Review.
-
Chromatin structure of pluripotent stem cells and induced pluripotent stem cells.Brief Funct Genomics. 2011 Jan;10(1):37-49. doi: 10.1093/bfgp/elq038. Brief Funct Genomics. 2011. PMID: 21325400 Free PMC article. Review.
-
SWI/SNF Chromatin Remodeling Enzymes in Melanoma.Epigenomes. 2022 Mar 18;6(1):10. doi: 10.3390/epigenomes6010010. Epigenomes. 2022. PMID: 35323214 Free PMC article. Review.
References
-
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Jiang J, Chan YS, Loh YH, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

