Function and specificity of synthetic Hox transcription factors in vivo
- PMID: 20147626
- PMCID: PMC2840133
- DOI: 10.1073/pnas.0914595107
Function and specificity of synthetic Hox transcription factors in vivo
Abstract
Homeotic (Hox) genes encode transcription factors that confer segmental identity along the anteroposterior axis of the embryo. However the molecular mechanisms underlying Hox-mediated transcription and the differential requirements for specificity in the regulation of the vast number of Hox-target genes remain ill-defined. Here we show that synthetic Sex combs reduced (Scr) genes that encode the Scr C terminus containing the homedomain (HD) and YPWM motif (Scr-HD) are functional in vivo. Synthetic Scr-HD peptides can induce ectopic salivary glands in the embryo and homeotic transformations in the adult fly, act as transcriptional activators and repressors during development, and participate in protein-protein interactions. Their transformation capacity was found to be enhanced over their full-length counterpart and mutations known to transform the full-length protein into constitutively active or inactive variants behaved accordingly in the synthetic peptides. Our results show that synthetic Scr-HD genes are sufficient for homeotic function in Drosophila and suggest that the N terminus of Scr has a role in transcriptional potency, rather than specificity. We also demonstrate that synthetic peptides behave largely in a predictable way, by exhibiting Scr-specific phenotypes throughout development, which makes them an important tool for synthetic biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
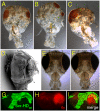
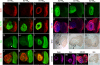
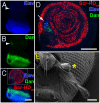
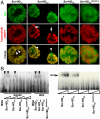
 under the control of dppblink-Gal4. Ubiquitously expressed mRFP1-tagged histone H2B was used to visualize chromatin. Scr-HDwt and Scr-HDAA readily associate with the chromosomes (as shown in the Green Channel) but also show sites of accumulation along the chromosome where loose chromatin compaction is shown as a low histone signal (Arrows). Arrowheads point at sites of high accumulation observed for Scr-HDwt and Scr-HDAA. The nucleus expressing the inactive Scr-HDDD shows some association of the transcription factor with the DNA, but it is also dispersed in the nucleoplasm. There is no pronounced banding pattern observed in this case, which suggests absence of specific binding. Scr-
under the control of dppblink-Gal4. Ubiquitously expressed mRFP1-tagged histone H2B was used to visualize chromatin. Scr-HDwt and Scr-HDAA readily associate with the chromosomes (as shown in the Green Channel) but also show sites of accumulation along the chromosome where loose chromatin compaction is shown as a low histone signal (Arrows). Arrowheads point at sites of high accumulation observed for Scr-HDwt and Scr-HDAA. The nucleus expressing the inactive Scr-HDDD shows some association of the transcription factor with the DNA, but it is also dispersed in the nucleoplasm. There is no pronounced banding pattern observed in this case, which suggests absence of specific binding. Scr- appears almost completely excluded from the chromosomes, mainly residing in the nucleoplasm. Scale bars in all cases are 20 μm. (B) Electrophoretic Mobility Shift Assay (EMSA) shows that only Scr-HDwt and Scr-HDAA bind DNA specifically in vitro. Both variants bound more strongly to BS2 than fkh250 (Left). Titration of peptide concentration (Right) revealed that even at high concentrations of transcription factor, Scr-HDDD and Scr-
appears almost completely excluded from the chromosomes, mainly residing in the nucleoplasm. Scale bars in all cases are 20 μm. (B) Electrophoretic Mobility Shift Assay (EMSA) shows that only Scr-HDwt and Scr-HDAA bind DNA specifically in vitro. Both variants bound more strongly to BS2 than fkh250 (Left). Titration of peptide concentration (Right) revealed that even at high concentrations of transcription factor, Scr-HDDD and Scr- do not bind DNA (BS2) specifically.
do not bind DNA (BS2) specifically.
Similar articles
-
Functional synthetic Antennapedia genes and the dual roles of YPWM motif and linker size in transcriptional activation and repression.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11959-64. doi: 10.1073/pnas.1108686108. Epub 2011 Jun 28. Proc Natl Acad Sci U S A. 2011. PMID: 21712439 Free PMC article.
-
Dimer formation via the homeodomain is required for function and specificity of Sex combs reduced in Drosophila.Dev Biol. 2012 Jul 1;367(1):78-89. doi: 10.1016/j.ydbio.2012.04.021. Epub 2012 Apr 28. Dev Biol. 2012. PMID: 22564794
-
Functional dissection of the mouse Hox-a5 gene.EMBO J. 1996 Mar 15;15(6):1313-22. EMBO J. 1996. PMID: 8635464 Free PMC article.
-
Understanding the genetic basis of morphological evolution: the role of homeotic genes in the diversification of the arthropod bauplan.Int J Dev Biol. 1998;42(3):453-61. Int J Dev Biol. 1998. PMID: 9654031 Review.
-
The specificity of homeotic gene function.Bioessays. 1995 Oct;17(10):855-63. doi: 10.1002/bies.950171007. Bioessays. 1995. PMID: 7487967 Review.
Cited by
-
Disentangling the many layers of eukaryotic transcriptional regulation.Annu Rev Genet. 2012;46:43-68. doi: 10.1146/annurev-genet-110711-155437. Epub 2012 Aug 28. Annu Rev Genet. 2012. PMID: 22934649 Free PMC article. Review.
-
Control of Hox transcription factor concentration and cell-to-cell variability by an auto-regulatory switch.Development. 2019 Jan 25;146(12):dev168179. doi: 10.1242/dev.168179. Development. 2019. PMID: 30642837 Free PMC article.
-
Developmental competence and the induction of ectopic proboscises in Drosophila melanogaster.Dev Genes Evol. 2013 Nov;223(6):375-387. doi: 10.1007/s00427-013-0454-8. Dev Genes Evol. 2013. PMID: 24121940
-
Variable motif utilization in homeotic selector (Hox)-cofactor complex formation controls specificity.Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21122-7. doi: 10.1073/pnas.1114118109. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160705 Free PMC article.
-
Dissecting the functional specificities of two Hox proteins.Genes Dev. 2010 Jul 15;24(14):1533-45. doi: 10.1101/gad.1936910. Genes Dev. 2010. PMID: 20634319 Free PMC article.
References
-
- Gehring WJ. Homeo boxes in the study of development. Science. 1987;236(4806):1245–1252. - PubMed
-
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–570. - PubMed
-
- Mann RS, Morata G. The developmental and molecular biology of genes that subdivide the body of Drosophila. Annu Rev Cell Dev Biol. 2000;16:243–271. - PubMed
-
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68(2):283–302. - PubMed
-
- Kappen C, Ruddle FH. Evolution of a regulatory gene family: HOM/HOX genes. Curr Opin Genet Dev. 1993;3(6):931–938. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

