Mature dendritic cells use endocytic receptors to capture and present antigens
- PMID: 20142498
- PMCID: PMC2840134
- DOI: 10.1073/pnas.0910609107
Mature dendritic cells use endocytic receptors to capture and present antigens
Abstract
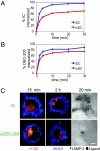
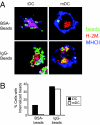
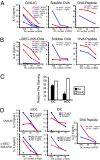
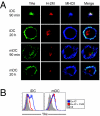
In response to inflammatory stimuli, dendritic cells (DCs) trigger the process of maturation, a terminal differentiation program required to initiate T-lymphocyte responses. A hallmark of maturation is down-regulation of endocytosis, which is widely assumed to restrict the ability of mature DCs to capture and present antigens encountered after the initial stimulus. We found that mature DCs continue to accumulate antigens, especially by receptor-mediated endocytosis and phagocytosis. Internalized antigens are transported normally to late endosomes and lysosomes, loaded onto MHC class II molecules (MHCII), and then presented efficiently to T cells. This occurs despite the fact that maturation results in the general depletion of MHCII from late endocytic compartments, with MHCII enrichment being typically thought to be a required feature of antigen processing and peptide loading compartments. Internalized antigens can also be cross-presented on MHC class I molecules, without any reduction in efficiency relative to immature DCs. Thus, although mature DCs markedly down-regulate their capacity for macropinocytosis, they continue to capture, process, and present antigens internalized via endocytic receptors, suggesting that they may continuously initiate responses to newly encountered antigens during the course of an infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis.Blood. 2004 Mar 15;103(6):2187-95. doi: 10.1182/blood-2003-08-2729. Epub 2003 Nov 6. Blood. 2004. PMID: 14604956
-
Dendritic cells continue to capture and present antigens after maturation in vivo.J Immunol. 2010 Aug 15;185(4):2140-6. doi: 10.4049/jimmunol.1000642. Epub 2010 Jul 19. J Immunol. 2010. PMID: 20644175 Free PMC article.
-
Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination.Immunity. 2006 Dec;25(6):885-94. doi: 10.1016/j.immuni.2006.11.001. Immunity. 2006. PMID: 17174123
-
Endosomal sorting of MHC class II determines antigen presentation by dendritic cells.Curr Opin Cell Biol. 2008 Aug;20(4):437-44. doi: 10.1016/j.ceb.2008.05.011. Epub 2008 Jul 5. Curr Opin Cell Biol. 2008. PMID: 18582577 Review.
-
Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells.Front Immunol. 2019 Jan 7;9:3098. doi: 10.3389/fimmu.2018.03098. eCollection 2018. Front Immunol. 2019. PMID: 30666258 Free PMC article. Review.
Cited by
-
Macropinocytosis in phagocytes: regulation of MHC class-II-restricted antigen presentation in dendritic cells.Front Physiol. 2015 Jan 30;6:1. doi: 10.3389/fphys.2015.00001. eCollection 2015. Front Physiol. 2015. PMID: 25688210 Free PMC article. Review.
-
Adipose cDC1s contribute to obesity-associated inflammation through STING-dependent IL-12 production.Nat Metab. 2023 Dec;5(12):2237-2252. doi: 10.1038/s42255-023-00934-4. Epub 2023 Nov 23. Nat Metab. 2023. PMID: 37996702
-
The Chemokine Receptor CCR7 Uses Distinct Signaling Modules With Biased Functionality to Regulate Dendritic Cells.Front Immunol. 2020 Apr 15;11:528. doi: 10.3389/fimmu.2020.00528. eCollection 2020. Front Immunol. 2020. PMID: 32351499 Free PMC article. Review.
-
Mannose and Mannose-6-Phosphate Receptor-Targeted Drug Delivery Systems and Their Application in Cancer Therapy.Adv Healthc Mater. 2018 Jul;7(14):e1701398. doi: 10.1002/adhm.201701398. Epub 2018 May 2. Adv Healthc Mater. 2018. PMID: 29719138 Free PMC article. Review.
-
Crosstalking with Dendritic Cells: A Path to Engineer Advanced T Cell Immunotherapy.Front Syst Biol. 2024;4:1372995. doi: 10.3389/fsysb.2024.1372995. Epub 2024 Apr 29. Front Syst Biol. 2024. PMID: 38911455 Free PMC article.
References
-
- Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. - PubMed
-
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. - PubMed
-
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. - PubMed
-
- Pierre P, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. - PubMed
-
- Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

