Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations
- PMID: 20142433
- PMCID: PMC2822606
- DOI: 10.1084/jem.20092506
Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations
Abstract
Mutations in isocitrate dehydrogenase 1 and 2 (IDH1/2), are present in most gliomas and secondary glioblastomas, but are rare in other neoplasms. IDH1/2 mutations are heterozygous, and affect a single arginine residue. Recently, IDH1 mutations were identified in 8% of acute myelogenous leukemia (AML) patients. A glioma study revealed that IDH1 mutations cause a gain-of-function, resulting in the production and accumulation of 2-hydroxyglutarate (2-HG). Genotyping of 145 AML biopsies identified 11 IDH1 R132 mutant samples. Liquid chromatography-mass spectrometry metabolite screening revealed increased 2-HG levels in IDH1 R132 mutant cells and sera, and uncovered two IDH2 R172K mutations. IDH1/2 mutations were associated with normal karyotypes. Recombinant IDH1 R132C and IDH2 R172K proteins catalyze the novel nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reduction of alpha-ketoglutarate (alpha-KG) to 2-HG. The IDH1 R132C mutation commonly found in AML reduces the affinity for isocitrate, and increases the affinity for NADPH and alpha-KG. This prevents the oxidative decarboxylation of isocitrate to alpha-KG, and facilitates the conversion of alpha-KG to 2-HG. IDH1/2 mutations confer an enzymatic gain of function that dramatically increases 2-HG in AML. This provides an explanation for the heterozygous acquisition of these mutations during tumorigenesis. 2-HG is a tractable metabolic biomarker of mutant IDH1/2 enzyme activity.
Figures
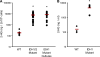

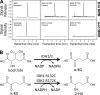
Comment in
-
Cancers converge at 2-HG.J Exp Med. 2010 Feb 15;207(2):265. doi: 10.1084/jem.2072iti5. Epub 2010 Feb 8. J Exp Med. 2010. PMID: 20142426 Free PMC article. No abstract available.
-
Metabolism and the leukemic stem cell.J Exp Med. 2010 Apr 12;207(4):677-80. doi: 10.1084/jem.20100523. Epub 2010 Apr 5. J Exp Med. 2010. PMID: 20368582 Free PMC article.
Similar articles
-
IDH mutations in glioma and acute myeloid leukemia.Trends Mol Med. 2010 Sep;16(9):387-97. doi: 10.1016/j.molmed.2010.07.002. Epub 2010 Aug 5. Trends Mol Med. 2010. PMID: 20692206 Review.
-
Replication Study: The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate.Elife. 2017 Jun 27;6:e26030. doi: 10.7554/eLife.26030. Elife. 2017. PMID: 28653623 Free PMC article.
-
Molecular alterations of isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) metabolic genes and additional genetic mutations in newly diagnosed acute myeloid leukemia patients.J Hematol Oncol. 2012 Mar 7;5:5. doi: 10.1186/1756-8722-5-5. J Hematol Oncol. 2012. PMID: 22397365 Free PMC article.
-
Serum 2-hydroxyglutarate production in IDH1- and IDH2-mutated de novo acute myeloid leukemia: a study by the Acute Leukemia French Association group.J Clin Oncol. 2014 Feb 1;32(4):297-305. doi: 10.1200/JCO.2013.50.2047. Epub 2013 Dec 16. J Clin Oncol. 2014. PMID: 24344214
-
Enasidenib and ivosidenib in AML.Minerva Med. 2020 Oct;111(5):411-426. doi: 10.23736/S0026-4806.20.07024-X. Epub 2020 Sep 21. Minerva Med. 2020. PMID: 32955829 Review.
Cited by
-
Targeting the Metabolic Paradigms in Cancer and Diabetes.Biomedicines. 2024 Jan 17;12(1):211. doi: 10.3390/biomedicines12010211. Biomedicines. 2024. PMID: 38255314 Free PMC article. Review.
-
Serum metabolomics analysis for early detection of colorectal cancer.J Gastroenterol. 2017 Jun;52(6):677-694. doi: 10.1007/s00535-016-1261-6. Epub 2016 Sep 20. J Gastroenterol. 2017. PMID: 27650200
-
Quantitative analysis of energy metabolic pathways in MCF-7 breast cancer cells by selected reaction monitoring assay.Mol Cell Proteomics. 2012 Aug;11(8):422-34. doi: 10.1074/mcp.M111.015214. Epub 2012 Apr 25. Mol Cell Proteomics. 2012. PMID: 22535206 Free PMC article.
-
Targeting histone methylation for cancer therapy: enzymes, inhibitors, biological activity and perspectives.J Hematol Oncol. 2016 Jun 17;9(1):49. doi: 10.1186/s13045-016-0279-9. J Hematol Oncol. 2016. PMID: 27316347 Free PMC article. Review.
-
Epigenetics in Cancer: A Hematological Perspective.PLoS Genet. 2016 Oct 10;12(10):e1006193. doi: 10.1371/journal.pgen.1006193. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27723796 Free PMC article. Review.
References
-
- Bleeker F.E., Lamba S., Leenstra S., Troost D., Hulsebos T., Vandertop W.P., Frattini M., Molinari F., Knowles M., Cerrato A., et al. 2009. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum. Mutat. 30:7–11 10.1002/humu.20937 - DOI - PubMed
-
- Ducray F., Marie Y., Sanson M. 2009. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360:2248. - PubMed
-
- Hartmann C., Meyer J., Balss J., Capper D., Mueller W., Christians A., Felsberg J., Wolter M., Mawrin C., Wick W., et al. 2009. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 118:469–474 10.1007/s00401-009-0561-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

