Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases
- PMID: 20139324
- PMCID: PMC2853420
- DOI: 10.1152/ajpheart.00252.2009
Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases
Abstract
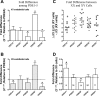
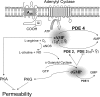
The importance of gonadal hormones in the regulation of vascular function has been documented. An alternate and essential contribution of the sex chromosomes to sex differences in vascular function is poorly understood. We reported previously sex differences in microvessel permeability (P(s)) responses to adenosine that were mediated by the cAMP signaling pathway (Wang J, PhD thesis, 2005; Wang J and Huxley V, Proceedings of the VIII World Congress of Microcirculation, 2007; Wang J and Huxley VH, Am J Physiol Heart Circ Physiol 291: H3094-H3105, 2006). The two cyclic nucleotides, cAMP and cGMP, central to the regulation of vascular barrier integrity, are hydrolyzed by phosphodiesterases (PDE). We hypothesized that microvascular endothelial cells (EC) would retain intrinsic and inheritable sexually dimorphic genes with respect to the PDEs modulating EC barrier function. Primary cultured microvascular EC from skeletal muscles isolated from male and female rats, respectively, were used. SRY (a sex-determining region Y gene) mRNA expression was observed exclusively in male, not female, cells. The predominant isoform among PDE1-5, present in both XY and XX EC, was PDE4. Expression mRNA levels of PDE1A (male > female) and PDE3B (male < female) were sex dependent; PDE2A, PDE4D, and PDE5A were sex independent. Barrier function, P(s), was determined from measures of albumin flux across confluent primary cultured microvessel XY and XX EC monolayers. Consistent with intact in situ microvessels, basal monolayer P(s) did not differ between XY (1.7 +/- 0.2 x 10(-6) cm/s; n = 8) and XX (1.8 +/- 0.1 x 10(-6) cm/s; n = 10) EC. Cilostazol, a PDE3 inhibitor, reduced (11%, P < 0.05) P(s) in XX, not XY, cells. These findings demonstrate the presence and maintenance of intrinsic sex-related differences in gene expression and cellular phenotype by microvascular EC in a gonadal-hormone-free environment. Furthermore, intrinsic cell-sex likely contributes significantly to sexual dimorphism in cardiovascular function.
Figures




Similar articles
-
Nitric oxide-induced changes in endothelial expression of phosphodiesterases 2, 3, and 5.Headache. 2010 Mar;50(3):431-41. doi: 10.1111/j.1526-4610.2009.01512.x. Epub 2009 Sep 14. Headache. 2010. PMID: 19751368
-
Differential effects of phosphodiesterase PDE-3/PDE-4-specific inhibitors on vasoconstriction and cAMP-dependent vasorelaxation following balloon angioplasty.Am J Physiol Heart Circ Physiol. 2007 Jun;292(6):H2973-81. doi: 10.1152/ajpheart.00419.2006. Epub 2007 Feb 9. Am J Physiol Heart Circ Physiol. 2007. PMID: 17293498
-
Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H786-93. doi: 10.1152/ajpheart.00349.2008. Epub 2008 Jun 27. Am J Physiol Heart Circ Physiol. 2008. PMID: 18586889 Free PMC article.
-
Cardiac Phosphodiesterases and Their Modulation for Treating Heart Disease.Handb Exp Pharmacol. 2017;243:249-269. doi: 10.1007/164_2016_82. Handb Exp Pharmacol. 2017. PMID: 27787716 Free PMC article. Review.
-
Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake.Cardiovasc Drug Rev. 2001 Winter;19(4):369-86. doi: 10.1111/j.1527-3466.2001.tb00076.x. Cardiovasc Drug Rev. 2001. PMID: 11830753 Review.
Cited by
-
Phosphodiesterase in heart and vessels: from physiology to diseases.Physiol Rev. 2024 Apr 1;104(2):765-834. doi: 10.1152/physrev.00015.2023. Epub 2023 Nov 16. Physiol Rev. 2024. PMID: 37971403 Free PMC article. Review.
-
Gender differences in response to cold pressor test assessed with velocity-encoded cardiovascular magnetic resonance of the coronary sinus.J Cardiovasc Magn Reson. 2011 Sep 23;13(1):54. doi: 10.1186/1532-429X-13-54. J Cardiovasc Magn Reson. 2011. PMID: 21943255 Free PMC article.
-
Multiple Genetic Associations with Irish Wolfhound Dilated Cardiomyopathy.Biomed Res Int. 2016;2016:6374082. doi: 10.1155/2016/6374082. Epub 2016 Dec 13. Biomed Res Int. 2016. PMID: 28070514 Free PMC article.
-
Crosstalk between high-density lipoproteins and endothelial cells in health and disease: Insights into sex-dependent modulation.Front Cardiovasc Med. 2022 Oct 6;9:989428. doi: 10.3389/fcvm.2022.989428. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36304545 Free PMC article. Review.
-
Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA.PLoS One. 2010 Dec 6;5(12):e15178. doi: 10.1371/journal.pone.0015178. PLoS One. 2010. PMID: 21151895 Free PMC article.
References
-
- Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol Heart Circ Physiol 274: H1885–H1894, 1998 - PubMed
-
- Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab 15: 6–11, 2004 - PubMed
-
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci 1007: 176–188, 2003 - PubMed
-
- Barber DA, Burnett JC, Jr, Fitzpatrick LA, Sieck GC, Miller VM. Gender and relaxation to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol 32: 5–11, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

