Novel forms of neurofascin 155 in the central nervous system: alterations in paranodal disruption models and multiple sclerosis
- PMID: 20129933
- PMCID: PMC2822635
- DOI: 10.1093/brain/awp341
Novel forms of neurofascin 155 in the central nervous system: alterations in paranodal disruption models and multiple sclerosis
Erratum in
- Brain. 2011 Apr;134(Pt 4):1250
Abstract
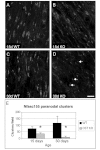
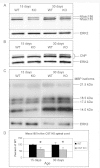
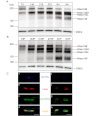
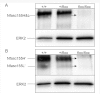
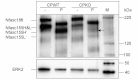
Stability of the myelin-axon unit is achieved, at least in part, by specialized paranodal junctions comprised of the neuronal heterocomplex of contactin and contactin-associated protein and the myelin protein neurofascin 155. In multiple sclerosis, normal distribution of these proteins is altered, resulting in the loss of the insulating myelin and consequently causing axonal dysfunction. Previously, this laboratory reported that mice lacking the myelin-enriched lipid sulphatide are characterized by a progressive deterioration of the paranodal structure. Here, it is shown that this deterioration is preceded by significant loss of neurofascin 155 clustering at the myelin paranode. Interestingly, prolonged electrophoretic separation revealed the existence of two neurofascin 155 bands, neurofascin 155 high and neurofascin 155 low, which are readily observed following N-linked deglycosylation. Neurofascin 155 high is observed at 7 days of age and reaches peak expression at one month of age, while neurofascin 155 low is first observed at 14 days of age and constantly increases until 5 months of age. Studies using conditional neurofascin knockout mice indicated that neurofascin 155 high and neurofascin 155 low are products of the neurofascin gene and are exclusively expressed by oligodendrocytes within the central nervous system. Neurofascin 155 high is a myelin paranodal protein while the distribution of neurofascin 155 low remains to be determined. While neurofascin 155 high levels are significantly reduced in the sulphatide null mice at 15 days, 30 days and 4 months of age, neurofascin 155 low levels remain unaltered. Although maintained at normal levels, neurofascin 155 low is incapable of preserving paranodal structure, thus indicating that neurofascin 155 high is required for paranodal stability. Additionally, comparisons between neurofascin 155 high and neurofascin 155 low in human samples revealed a significant alteration, specifically in multiple sclerosis plaques.
Figures



Similar articles
-
Neurofascins are required to establish axonal domains for saltatory conduction.Neuron. 2005 Dec 8;48(5):737-42. doi: 10.1016/j.neuron.2005.10.019. Neuron. 2005. PMID: 16337912
-
Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis.Brain. 2006 Dec;129(Pt 12):3173-85. doi: 10.1093/brain/awl290. Epub 2006 Oct 14. Brain. 2006. PMID: 17041241
-
In vivo deletion of immunoglobulin domains 5 and 6 in neurofascin (Nfasc) reveals domain-specific requirements in myelinated axons.J Neurosci. 2010 Apr 7;30(14):4868-76. doi: 10.1523/JNEUROSCI.5951-09.2010. J Neurosci. 2010. PMID: 20371806 Free PMC article.
-
Anti-neurofascin autoantibody and demyelination.Neurochem Int. 2019 Nov;130:104360. doi: 10.1016/j.neuint.2018.12.011. Epub 2018 Dec 22. Neurochem Int. 2019. PMID: 30582947 Review.
-
Organisation and control of neuronal connectivity and myelination by cell adhesion molecule neurofascin.Adv Neurobiol. 2014;8:231-47. doi: 10.1007/978-1-4614-8090-7_10. Adv Neurobiol. 2014. PMID: 25300139 Review.
Cited by
-
Novel molecular insights into the critical role of sulfatide in myelin maintenance/function.J Neurochem. 2016 Oct;139(1):40-54. doi: 10.1111/jnc.13738. Epub 2016 Aug 15. J Neurochem. 2016. PMID: 27417284 Free PMC article.
-
Altered potassium channel distribution and composition in myelinated axons suppresses hyperexcitability following injury.Elife. 2016 Apr 19;5:e12661. doi: 10.7554/eLife.12661. Elife. 2016. PMID: 27033551 Free PMC article.
-
Adult-onset depletion of sulfatide leads to axonal degeneration with relative myelin sparing.Glia. 2023 Sep;71(9):2285-2303. doi: 10.1002/glia.24423. Epub 2023 Jun 7. Glia. 2023. PMID: 37283058 Free PMC article.
-
Nfasc155H and MAG are specifically susceptible to detergent extraction in the absence of the myelin sphingolipid sulfatide.Neurochem Res. 2013 Dec;38(12):2490-502. doi: 10.1007/s11064-013-1162-5. Epub 2013 Oct 2. Neurochem Res. 2013. PMID: 24081651 Free PMC article.
-
Focal loss of the paranodal domain protein Neurofascin155 in the internal capsule impairs cortically induced muscle activity in vivo.Mol Brain. 2020 Nov 23;13(1):159. doi: 10.1186/s13041-020-00698-y. Mol Brain. 2020. PMID: 33228720 Free PMC article.
References
-
- Arvanitis DN, Min W, Gong Y, Heng YM, Boggs JM. Two types of detergent-insoluble, glycosphingolipid/cholesterol-rich membrane domains from isolated myelin. J Neurochem. 2005;94:1696–710. - PubMed
-
- Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–61. - PubMed
-
- Basak S, Raju K, Babiarz J, Kane-Goldsmith N, Koticha D, Grumet M. Differential expression and functions of neuronal and glial neurofascin isoforms and splice variants during PNS development. Dev Biol. 2007;311:408–22. - PubMed
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

