Guardian of corpulence: a hypothesis on p53 signaling in the fat cell
- PMID: 20126301
- PMCID: PMC2746679
- DOI: 10.2217/clp.09.2
Guardian of corpulence: a hypothesis on p53 signaling in the fat cell
Abstract
Adipocytes provide an organism with fuel in times of caloric deficit, and are an important type of endocrine cell in the maintenance of metabolic homeostasis. In addition, as a lipid-sink, adipocytes serve an equally important role in the protection of organs from the damaging effects of ectopic lipid deposition. For the organism, it is of vital importance to maintain adipocyte viability, yet the fat depot is a demanding extracellular environment with high levels of interstitial free fatty acids and associated lipotoxic effects. These surroundings are less than beneficial for the overall health of any resident cell, adipocyte and preadipocyte alike. In this review, we discuss the process of adipogenesis and the potential involvement of the p53 tumor-suppressor protein in alleviating some of the cellular stress experienced by these cells. In particular, we discuss p53-mediated mechanisms that prevent damage caused by reactive oxygen species and the effects of lipotoxicity. We also suggest the potential for two p53 target genes, START domain-containing protein 4 (StARD4) and oxysterol-binding protein (OSBP), with the concomitant synthesis of the signaling molecule oxysterol, to participate in adipogenesis.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Figures

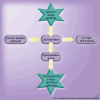
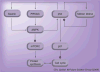

Similar articles
-
Omega-3 fatty acids and adipose tissue biology.Mol Aspects Med. 2018 Dec;64:147-160. doi: 10.1016/j.mam.2018.01.004. Epub 2018 Jan 17. Mol Aspects Med. 2018. PMID: 29329795 Review.
-
The Intricate Role of p53 in Adipocyte Differentiation and Function.Cells. 2020 Dec 7;9(12):2621. doi: 10.3390/cells9122621. Cells. 2020. PMID: 33297294 Free PMC article. Review.
-
Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance.Biochimie. 2013 Mar;95(3):585-94. doi: 10.1016/j.biochi.2012.12.014. Epub 2012 Dec 26. Biochimie. 2013. PMID: 23274128 Review.
-
Adipogenesis in fish.J Exp Biol. 2018 Mar 7;221(Pt Suppl 1):jeb161588. doi: 10.1242/jeb.161588. J Exp Biol. 2018. PMID: 29514876 Review.
-
FFAs-ROS-ERK/P38 pathway plays a key role in adipocyte lipotoxicity on osteoblasts in co-culture.Biochimie. 2014 Jun;101:123-31. doi: 10.1016/j.biochi.2014.01.002. Epub 2014 Jan 11. Biochimie. 2014. PMID: 24424405
Cited by
-
Differential association of S100A9, an inflammatory marker, and p53, a cell cycle marker, expression with epicardial adipocyte size in patients with cardiovascular disease.Inflammation. 2014 Oct;37(5):1504-12. doi: 10.1007/s10753-014-9876-3. Inflammation. 2014. PMID: 24700313
-
Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer.Mol Metab. 2020 Mar;33:2-22. doi: 10.1016/j.molmet.2019.10.002. Epub 2019 Oct 18. Mol Metab. 2020. PMID: 31685430 Free PMC article. Review.
-
Coordination of the AMPK, Akt, mTOR, and p53 Pathways under Glucose Starvation.Int J Mol Sci. 2022 Nov 29;23(23):14945. doi: 10.3390/ijms232314945. Int J Mol Sci. 2022. PMID: 36499271 Free PMC article.
-
Sirt7 promotes adipogenesis in the mouse by inhibiting autocatalytic activation of Sirt1.Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8352-E8361. doi: 10.1073/pnas.1706945114. Epub 2017 Sep 18. Proc Natl Acad Sci U S A. 2017. PMID: 28923965 Free PMC article.
-
Prediabetic changes in gene expression induced by aspartame and monosodium glutamate in Trans fat-fed C57Bl/6 J mice.Nutr Metab (Lond). 2013 Jun 19;10:44. doi: 10.1186/1743-7075-10-44. eCollection 2013. Nutr Metab (Lond). 2013. PMID: 23783067 Free PMC article.
References
-
- Arner P. Insulin resistance in Type 2 diabetes – role of the adipokines. Curr Mol Med. 2005;5(3):333–339. - PubMed
-
- Chen X, Cushman SW, Pannell LK, Hess S. Quantitative proteomic analysis of the secretory proteins from rat adipose cells using a 2D liquid chromatography-MS/MS approach. J Proteome Res. 2005;4(2):570–577. - PubMed
-
- Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, Type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8(5):395–408. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
