Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections
- PMID: 20121613
- PMCID: PMC2819217
- DOI: 10.5858/134.2.235
Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections
Abstract
Context: In March 2009, a novel swine-origin influenza A/H1N1 virus was identified. After global spread, the World Health Organization in June declared the first influenza pandemic in 41 years.
Objective: To describe the clinicopathologic characteristics of 34 people who died following confirmed A/H1N1 infection with emphasis on the pulmonary pathology findings.
Design: We reviewed medical records, autopsy reports, microbiologic studies, and microscopic slides of 34 people who died between May 15 and July 9, 2009, and were investigated either by the New York City Office of Chief Medical Examiner (32 deaths) or through the consultation service of a coauthor (2 deaths).
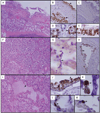
Results: Most of the 34 decedents (62%) were between 25 and 49 years old (median, 41.5 years). Tracheitis, bronchiolitis, and diffuse alveolar damage were noted in most cases. Influenza viral antigen was observed most commonly in the epithelium of the tracheobronchial tree but also in alveolar epithelial cells and macrophages. Most cases were reverse transcription-polymerase chain reaction positive for influenza. Histologic and microbiologic autopsy evidence of bacterial pneumonia was detected in 55% of cases. Underlying medical conditions including cardiorespiratory diseases and immunosuppression were present in 91% of cases. Obesity (body mass index, >30) was noted in 72% of adult and adolescent cases.
Conclusions: The pulmonary pathologic findings in fatal disease caused by the novel pandemic influenza virus are similar to findings identified in past pandemics. Superimposed bacterial infections of the respiratory tract were common. Preexisting obesity, cardiorespiratory diseases, and other comorbidities also were prominent findings among the decedents.
Figures
Similar articles
-
Predictive clinicopathological features derived from systematic autopsy examination of patients who died with A/H1N1 influenza infection in the UK 2009-10 pandemic.Health Technol Assess. 2010 Dec;14(55):83-114. doi: 10.3310/hta14550-02. Health Technol Assess. 2010. PMID: 21208548
-
Histopathological and immunohistochemical findings of 20 autopsy cases with 2009 H1N1 virus infection.Mod Pathol. 2012 Jan;25(1):1-13. doi: 10.1038/modpathol.2011.125. Epub 2011 Aug 26. Mod Pathol. 2012. PMID: 21874012
-
Postmortem findings in eight cases of influenza A/H1N1.Mod Pathol. 2010 Nov;23(11):1449-57. doi: 10.1038/modpathol.2010.148. Epub 2010 Aug 27. Mod Pathol. 2010. PMID: 20802471
-
Pandemic novel 2009 H1N1 influenza: what have we learned?Semin Respir Crit Care Med. 2011 Aug;32(4):393-9. doi: 10.1055/s-0031-1283279. Epub 2011 Aug 19. Semin Respir Crit Care Med. 2011. PMID: 21858744 Review.
-
Weight and prognosis for influenza A(H1N1)pdm09 infection during the pandemic period between 2009 and 2011: a systematic review of observational studies with meta-analysis.Infect Dis (Lond). 2016 Nov-Dec;48(11-12):813-22. doi: 10.1080/23744235.2016.1201721. Epub 2016 Jul 6. Infect Dis (Lond). 2016. PMID: 27385315 Review.
Cited by
-
Reciprocal impacts of obesity and coronavirus disease 2019.J Res Med Sci. 2020 Dec 30;25:110. doi: 10.4103/jrms.JRMS_416_20. eCollection 2020. J Res Med Sci. 2020. PMID: 33912220 Free PMC article. No abstract available.
-
Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity.Am J Physiol Lung Cell Mol Physiol. 2012 May 15;302(10):L1067-77. doi: 10.1152/ajplung.00190.2011. Epub 2012 Mar 2. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22387293 Free PMC article.
-
Bacterial complications of respiratory tract viral illness: a comprehensive evaluation.J Infect Dis. 2013 Aug 1;208(3):432-41. doi: 10.1093/infdis/jit190. Epub 2013 May 9. J Infect Dis. 2013. PMID: 23661797 Free PMC article.
-
Innate immune response of human alveolar macrophages during influenza A infection.PLoS One. 2012;7(3):e29879. doi: 10.1371/journal.pone.0029879. Epub 2012 Mar 2. PLoS One. 2012. PMID: 22396727 Free PMC article.
-
The "forgotten zone": acquired disorders of the trachea in adults.Respir Med. 2013 Sep;107(9):1301-13. doi: 10.1016/j.rmed.2013.03.017. Epub 2013 May 10. Respir Med. 2013. PMID: 23669413 Free PMC article. Review.
References
-
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. - PubMed
-
- Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76(1):105–115. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

