Carbohydrate engineered cells for regenerative medicine
- PMID: 20117158
- PMCID: PMC3032398
- DOI: 10.1016/j.addr.2010.01.003
Carbohydrate engineered cells for regenerative medicine
Abstract
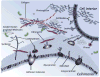
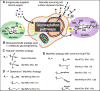
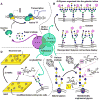
Carbohydrates are integral components of the stem cell niche on several levels; proteoglycans are a major constituent of the extracellular matrix (ECM) surrounding a cell, glycosoaminoglycans (GAGs) help link cells to the ECM and the neighboring cells, and small but informationally-rich oligosaccharides provide a "sugar code" that identifies each cell and provides it with unique functions. This article samples roles that glycans play in development and then describes how metabolic glycoengineering - a technique where monosaccharide analogs are introduced into the metabolic pathways of a cell and are biosynthetically incorporated into the glycocalyx - is overcoming many of the long-standing barriers to manipulating carbohydrates in living cells and tissues and is becoming an intriguing new tool for tissue engineering and regenerative medicine.
2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering.Adv Healthc Mater. 2014 May;3(5):628-41. doi: 10.1002/adhm.201300620. Epub 2014 Jan 20. Adv Healthc Mater. 2014. PMID: 24443420 Free PMC article. Review.
-
Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances.Biomed Res Int. 2014;2014:921905. doi: 10.1155/2014/921905. Epub 2014 Jul 20. Biomed Res Int. 2014. PMID: 25143954 Free PMC article. Review.
-
Employing Extracellular Matrix-Based Tissue Engineering Strategies for Age-Dependent Tissue Degenerations.Int J Mol Sci. 2021 Aug 29;22(17):9367. doi: 10.3390/ijms22179367. Int J Mol Sci. 2021. PMID: 34502277 Free PMC article. Review.
-
Decellularized Extracellular Matrix for Tissue Engineering (Review).Sovrem Tekhnologii Med. 2022;14(3):57-68. doi: 10.17691/stm2022.14.3.07. Epub 2022 May 28. Sovrem Tekhnologii Med. 2022. PMID: 37064810 Free PMC article. Review.
-
Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine.Curr Stem Cell Res Ther. 2017;12(3):233-246. doi: 10.2174/1574888X11666160905092513. Curr Stem Cell Res Ther. 2017. PMID: 27593448 Review.
Cited by
-
Biomimetic electrospun nanofibrous structures for tissue engineering.Mater Today (Kidlington). 2013 Jun 1;16(6):229-241. doi: 10.1016/j.mattod.2013.06.005. Mater Today (Kidlington). 2013. PMID: 25125992 Free PMC article.
-
Three-dimensional scaffold from decellularized human gingiva for cell cultures: glycoconjugates and cell behavior.Cell J. 2013 Summer;15(2):166-75. Epub 2013 Jul 2. Cell J. 2013. PMID: 23862119 Free PMC article.
-
Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration.Biomater Sci. 2018 May 29;6(6):1299-1311. doi: 10.1039/c8bm00260f. Biomater Sci. 2018. PMID: 29725688 Free PMC article. Review.
-
Programming Cell-Cell Interactions through Non-genetic Membrane Engineering.Cell Chem Biol. 2018 Aug 16;25(8):931-940. doi: 10.1016/j.chembiol.2018.05.009. Epub 2018 Jun 14. Cell Chem Biol. 2018. PMID: 29909993 Free PMC article. Review.
-
Electrospinning of Cross-Linked Magnetic Chitosan Nanofibers for Protein Release.AAPS PharmSciTech. 2015 Dec;16(6):1480-6. doi: 10.1208/s12249-015-0336-7. Epub 2015 May 29. AAPS PharmSciTech. 2015. PMID: 26022546 Free PMC article.
References
-
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. - PubMed
-
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. - PubMed
-
- Esko JD, Kimata K, Lindahl U. Chapter 16: Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Woodbury, New York: Cold Spring Harbor Press; 2009. - PubMed
-
- Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa. Tiss Eng. 1996;2:209–217. - PubMed
-
- Martin GR, Timpl R, Kuhn K. Basement-membrane proteins - molecular structure and function. Ad Prot Chem. 1988;39:1–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

