Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes
- PMID: 20103727
- PMCID: PMC2818090
- DOI: 10.1158/1940-6207.CAPR-09-0044
Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes
Abstract
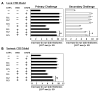
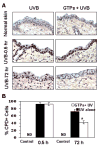
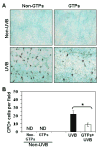
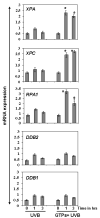
UV radiation-induced immunosuppression has been implicated in the development of skin cancers. Green tea polyphenols (GTP) in drinking water prevent photocarcinogenesis in the skin of mice. We studied whether GTPs in drinking water (0.1-0.5%, w/v) prevent UV-induced immunosuppression and (if so) potential mechanisms of this effect in mice. GTPs (0.2% and 0.5%, w/v) reduced UV-induced suppression of contact hypersensitivity (CHS) in response to a contact sensitizer in local (58-62% reductions; P < 0.001) and systemic (51-55% reductions; P < 0.005) models of CHS. Compared with untreated mice, GTP-treated mice (0.2%, w/v) had a reduced number of cyclobutane pyrimidine dimer-positive (CPD(+)) cells (59%; P < 0.001) in the skin, showing faster repair of UV-induced DNA damage, and had a reduced (2-fold) migration of CPD(+) cells from the skin to draining lymph nodes, which was associated with elevated levels of nucleotide excision repair (NER) genes. GTPs did not prevent UV-induced immunosuppression in NER-deficient mice but significantly prevented it in NER-proficient mice (P < 0.001); immunohistochemical analysis of CPD(+) cells indicated that GTPs reduced the numbers of UV-induced CPD(+) cells in NER-proficient mice (P < 0.001) but not in NER-deficient mice. Southwestern dot-blot analysis revealed that GTPs repaired UV-induced CPDs in xeroderma pigmentosum complementation group A (XPA)-proficient cells of a healthy person but did not in XPA-deficient cells obtained from XPA patients, indicating that a NER mechanism is involved in DNA repair. This study is the first to show a novel NER mechanism by which drinking GTPs prevents UV-induced immunosuppression and that inhibiting UV-induced immunosuppression may underlie the chemopreventive activity of GTPs against photocarcinogenesis.
Conflict of interest statement
Figures


Comment in
-
The promise of natural products for blocking early events in skin carcinogenesis.Cancer Prev Res (Phila). 2010 Feb;3(2):132-5. doi: 10.1158/1940-6207.CAPR-09-0267. Epub 2010 Jan 26. Cancer Prev Res (Phila). 2010. PMID: 20103730 Review.
Similar articles
-
Prevention of ultraviolet radiation-induced immunosuppression by (-)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair.Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2272-80. doi: 10.1158/1078-0432.CCR-05-2672. Clin Cancer Res. 2006. PMID: 16609044
-
Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum group A-dependent mechanism.Cancer Prev Res (Phila). 2010 Dec;3(12):1621-9. doi: 10.1158/1940-6207.CAPR-10-0137. Epub 2010 Oct 8. Cancer Prev Res (Phila). 2010. PMID: 20947490
-
(-)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair.Cancer Res. 2006 May 15;66(10):5512-20. doi: 10.1158/0008-5472.CAN-06-0218. Cancer Res. 2006. Retraction in: Cancer Res. 2018 Dec 1;78(23):6709. doi: 10.1158/0008-5472.CAN-18-3092 PMID: 16707481 Retracted.
-
Green tea polyphenols: DNA photodamage and photoimmunology.J Photochem Photobiol B. 2001 Dec 31;65(2-3):109-14. doi: 10.1016/s1011-1344(01)00248-2. J Photochem Photobiol B. 2001. PMID: 11809367 Review.
-
Green tea prevents non-melanoma skin cancer by enhancing DNA repair.Arch Biochem Biophys. 2011 Apr 15;508(2):152-8. doi: 10.1016/j.abb.2010.11.015. Epub 2010 Nov 19. Arch Biochem Biophys. 2011. PMID: 21094124 Free PMC article. Review.
Cited by
-
Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats.Antioxidants (Basel). 2021 Nov 28;10(12):1906. doi: 10.3390/antiox10121906. Antioxidants (Basel). 2021. PMID: 34943009 Free PMC article.
-
Protective mechanisms of green tea polyphenols in skin.Oxid Med Cell Longev. 2012;2012:560682. doi: 10.1155/2012/560682. Epub 2012 Jun 26. Oxid Med Cell Longev. 2012. PMID: 22792414 Free PMC article. Review.
-
Effects of dietary fish oil on the depletion of carcinogenic PAH-DNA adduct levels in the liver of B6C3F1 mouse.PLoS One. 2011;6(10):e26589. doi: 10.1371/journal.pone.0026589. Epub 2011 Oct 31. PLoS One. 2011. PMID: 22066002 Free PMC article.
-
The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution.Oxid Med Cell Longev. 2014;2014:671539. doi: 10.1155/2014/671539. Epub 2014 Jul 20. Oxid Med Cell Longev. 2014. PMID: 25140198 Free PMC article. Review.
-
Polyphenols and DNA Damage: A Mixed Blessing.Nutrients. 2016 Dec 3;8(12):785. doi: 10.3390/nu8120785. Nutrients. 2016. PMID: 27918471 Free PMC article. Review.
References
-
- Katiyar SK, Mukhtar H. Tea in chemoprevention of cancer: Epidemiologic and experimental studies. Int J Oncol. 1996;8:221–38. - PubMed
-
- Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. - PubMed
-
- Wang ZY, Huang MT, Ferraro T, et al. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992;52:1162–70. - PubMed
-
- Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–7. - PubMed
-
- Toews GB, Bergstresser PR, Streilein JW, Sullivan S. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

