Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells
- PMID: 20098744
- PMCID: PMC2808253
- DOI: 10.1371/journal.pone.0008774
Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells
Abstract
Background: Migrating leukocytes normally have a polarized morphology with an actin-rich lamellipodium at the front and a uropod at the rear. Microtubules (MTs) are required for persistent migration and chemotaxis, but how they affect cell polarity is not known.
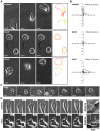
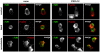
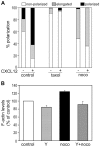
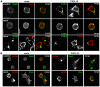
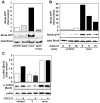
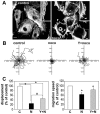
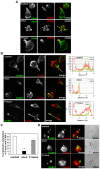
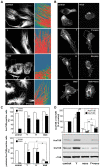
Methodology/principal findings: Here we report that T cells treated with nocodazole to disrupt MTs are unable to form a stable uropod or lamellipodium, and instead often move by membrane blebbing with reduced migratory persistence. However, uropod-localized receptors and ezrin/radixin/moesin proteins still cluster in nocodazole-treated cells, indicating that MTs are required specifically for uropod stability. Nocodazole stimulates RhoA activity, and inhibition of the RhoA target ROCK allows nocodazole-treated cells to re-establish lamellipodia and uropods and persistent migratory polarity. ROCK inhibition decreases nocodazole-induced membrane blebbing and stabilizes MTs. The myosin inhibitor blebbistatin also stabilizes MTs, indicating that RhoA/ROCK act through myosin II to destabilize MTs.
Conclusions/significance: Our results indicate that RhoA/ROCK signaling normally contributes to migration by affecting both actomyosin contractility and MT stability. We propose that regulation of MT stability and RhoA/ROCK activity is a mechanism to alter T-cell migratory behavior from lamellipodium-based persistent migration to bleb-based migration with frequent turning.
Conflict of interest statement
Figures

Similar articles
-
Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network.J Biol Chem. 2010 Oct 8;285(41):31661-71. doi: 10.1074/jbc.M110.145037. Epub 2010 Aug 3. J Biol Chem. 2010. PMID: 20682776 Free PMC article.
-
Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation.J Cell Biol. 2004 Oct 25;167(2):327-37. doi: 10.1083/jcb.200403091. J Cell Biol. 2004. PMID: 15504914 Free PMC article.
-
Combretastatin (CA)-4 and its novel analogue CA-432 impair T-cell migration through the Rho/ROCK signalling pathway.Biochem Pharmacol. 2014 Dec 15;92(4):544-57. doi: 10.1016/j.bcp.2014.10.002. Epub 2014 Oct 22. Biochem Pharmacol. 2014. PMID: 25450669
-
Functions of Rho family of small GTPases and Rho-associated coiled-coil kinases in bone cells during differentiation and mineralization.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):1009-1023. doi: 10.1016/j.bbagen.2017.02.005. Epub 2017 Feb 8. Biochim Biophys Acta Gen Subj. 2017. PMID: 28188861 Review.
-
Dynamic functions of RhoA in tumor cell migration and invasion.Small GTPases. 2013 Jul-Sep;4(3):141-7. doi: 10.4161/sgtp.25131. Epub 2013 Jun 10. Small GTPases. 2013. PMID: 24025634 Free PMC article. Review.
Cited by
-
Human neutrophil cytoskeletal dynamics and contractility actively contribute to trans-endothelial migration.PLoS One. 2013 Apr 23;8(4):e61377. doi: 10.1371/journal.pone.0061377. Print 2013. PLoS One. 2013. PMID: 23626676 Free PMC article.
-
Endothelial repair in stented arteries is accelerated by inhibition of Rho-associated protein kinase.Cardiovasc Res. 2016 Dec;112(3):689-701. doi: 10.1093/cvr/cvw210. Epub 2016 Sep 26. Cardiovasc Res. 2016. PMID: 27671802 Free PMC article.
-
Integrin crosstalk allows CD4+ T lymphocytes to continue migrating in the upstream direction after flow.Integr Biol (Camb). 2019 Dec 31;11(10):384-393. doi: 10.1093/intbio/zyz034. Integr Biol (Camb). 2019. PMID: 31851360 Free PMC article.
-
p120ctn and P-cadherin but not E-cadherin regulate cell motility and invasion of DU145 prostate cancer cells.PLoS One. 2010 Jul 27;5(7):e11801. doi: 10.1371/journal.pone.0011801. PLoS One. 2010. PMID: 20668551 Free PMC article.
-
Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network.J Biol Chem. 2010 Oct 8;285(41):31661-71. doi: 10.1074/jbc.M110.145037. Epub 2010 Aug 3. J Biol Chem. 2010. PMID: 20682776 Free PMC article.
References
-
- Hogg N, Smith A, McDowall A, Giles K, Stanley P, et al. How T cells use LFA-1 to attach and migrate. Immunol Lett. 2004;92:51–54. - PubMed
-
- Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

