Influence of the NR3A subunit on NMDA receptor functions
- PMID: 20097255
- PMCID: PMC2883719
- DOI: 10.1016/j.pneurobio.2010.01.004
Influence of the NR3A subunit on NMDA receptor functions
Abstract
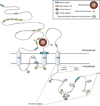
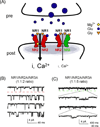
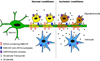
Various combinations of subunits assemble to form the NMDA-type glutamate receptor (NMDAR), generating diversity in its functions. Here we review roles of the unique NMDAR subunit, NR3A, which acts in a dominant-negative manner to suppress receptor activity. NR3A-containing NMDARs display striking regional and temporal expression specificity, and, unlike most other NMDAR subtypes, they have a low conductance, are only modestly permeable to Ca(2+), and pass current at hyperpolarized potentials in the presence of magnesium. While glutamate activates triheteromeric NMDARs composed of NR1/NR2/NR3A subunits, glycine is sufficient to activate diheteromeric NR1/NR3A-containing receptors. NR3A dysfunction may contribute to neurological disorders involving NMDARs, and the subunit offers an attractive therapeutic target given its distinct pharmacological and structural properties.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits.Nature. 2002 Feb 14;415(6873):793-8. doi: 10.1038/nature715. Epub 2002 Jan 30. Nature. 2002. PMID: 11823786
-
NR3A modulates the outer vestibule of the "NMDA" receptor channel.J Neurosci. 2006 Dec 20;26(51):13156-66. doi: 10.1523/JNEUROSCI.2552-06.2006. J Neurosci. 2006. PMID: 17182766 Free PMC article.
-
Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons.J Neurophysiol. 2008 Jan;99(1):122-32. doi: 10.1152/jn.01044.2006. Epub 2007 Nov 14. J Neurophysiol. 2008. PMID: 18003876 Free PMC article.
-
NMDA receptor subunits: function and pharmacology.Curr Opin Pharmacol. 2007 Feb;7(1):39-47. doi: 10.1016/j.coph.2006.08.011. Epub 2006 Nov 7. Curr Opin Pharmacol. 2007. PMID: 17088105 Review.
-
Shuffling the deck anew: how NR3 tweaks NMDA receptor function.Mol Neurobiol. 2008 Aug;38(1):16-26. doi: 10.1007/s12035-008-8029-9. Epub 2008 Jul 25. Mol Neurobiol. 2008. PMID: 18654865 Review.
Cited by
-
Retinoic acid-mediated homeostatic plasticity in the nucleus accumbens core contributes to incubation of cocaine craving.Psychopharmacology (Berl). 2024 Oct;241(10):1983-2001. doi: 10.1007/s00213-024-06612-x. Epub 2024 Jun 27. Psychopharmacology (Berl). 2024. PMID: 38935096
-
Drug-evoked plasticity: do addictive drugs reopen a critical period of postnatal synaptic development?Front Mol Neurosci. 2012 Jun 15;5:75. doi: 10.3389/fnmol.2012.00075. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22715323 Free PMC article.
-
Synaptic neurotransmitter-gated receptors.Cold Spring Harb Perspect Biol. 2012 Mar 1;4(3):a009662. doi: 10.1101/cshperspect.a009662. Cold Spring Harb Perspect Biol. 2012. PMID: 22233560 Free PMC article. Review.
-
Oligodendrocyte N-methyl-D-aspartate receptor signaling: insights into its functions.Mol Neurobiol. 2013 Apr;47(2):845-56. doi: 10.1007/s12035-013-8408-8. Epub 2013 Jan 24. Mol Neurobiol. 2013. PMID: 23345133 Review.
-
Presynaptic NMDA Receptors Influence Ca2+ Dynamics by Interacting with Voltage-Dependent Calcium Channels during the Induction of Long-Term Depression.Neural Plast. 2022 Feb 7;2022:2900875. doi: 10.1155/2022/2900875. eCollection 2022. Neural Plast. 2022. PMID: 35178084 Free PMC article.
References
-
- Al-Hallaq RA, Jarabek BR, Fu Z, Vicini S, Wolfe BB, Yasuda RP. Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Mol Pharmacol. 2002;62:1119–1127. - PubMed
-
- Andersson O, Stenqvist A, Attersand A, von Euler G. Nucleotide sequence, genomic organization, and chromosomal localization of genes encoding the human NMDA receptor subunits NR3A and NR3B. Genomics. 2001;78:178–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

