Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential
- PMID: 20095865
- PMCID: PMC2935339
- DOI: 10.1089/ars.2009.2876
Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential
Abstract
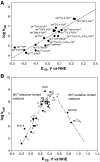
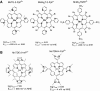
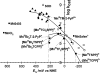
Oxidative stress has become widely viewed as an underlying condition in a number of diseases, such as ischemia-reperfusion disorders, central nervous system disorders, cardiovascular conditions, cancer, and diabetes. Thus, natural and synthetic antioxidants have been actively sought. Superoxide dismutase is a first line of defense against oxidative stress under physiological and pathological conditions. Therefore, the development of therapeutics aimed at mimicking superoxide dismutase was a natural maneuver. Metalloporphyrins, as well as Mn cyclic polyamines, Mn salen derivatives and nitroxides were all originally developed as SOD mimics. The same thermodynamic and electrostatic properties that make them potent SOD mimics may allow them to reduce other reactive species such as peroxynitrite, peroxynitrite-derived CO(3)(*-), peroxyl radical, and less efficiently H(2)O(2). By doing so SOD mimics can decrease both primary and secondary oxidative events, the latter arising from the inhibition of cellular transcriptional activity. To better judge the therapeutic potential and the advantage of one over the other type of compound, comparative studies of different classes of drugs in the same cellular and/or animal models are needed. We here provide a comprehensive overview of the chemical properties and some in vivo effects observed with various classes of compounds with a special emphasis on porphyrin-based compounds.
Figures




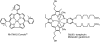
Comment in
-
Superoxide dismutase mimics.Antioxid Redox Signal. 2011 Mar 15;14(6):1173; author reply 1174-6. doi: 10.1089/ars.2010.3758. Antioxid Redox Signal. 2011. PMID: 21110789 Free PMC article. No abstract available.
Similar articles
-
Superoxide dismutase mimics.Antioxid Redox Signal. 2011 Mar 15;14(6):1173; author reply 1174-6. doi: 10.1089/ars.2010.3758. Antioxid Redox Signal. 2011. PMID: 21110789 Free PMC article. No abstract available.
-
Potential Therapeutic Applications of MnSODs and SOD-Mimetics.Chemistry. 2018 Apr 6;24(20):5032-5041. doi: 10.1002/chem.201704561. Epub 2017 Dec 12. Chemistry. 2018. PMID: 29131419 Review.
-
High lipophilicity of meta Mn(III) N-alkylpyridylporphyrin-based superoxide dismutase mimics compensates for their lower antioxidant potency and makes them as effective as ortho analogues in protecting superoxide dismutase-deficient Escherichia coli.J Med Chem. 2009 Dec 10;52(23):7868-72. doi: 10.1021/jm900576g. J Med Chem. 2009. PMID: 19954250 Free PMC article.
-
Only one of a wide assortment of manganese-containing SOD mimicking compounds rescues the slow aerobic growth phenotypes of both Escherichia coli and Saccharomyces cerevisiae strains lacking superoxide dismutase enzymes.J Inorg Biochem. 2007 Nov;101(11-12):1875-82. doi: 10.1016/j.jinorgbio.2007.07.008. Epub 2007 Jul 16. J Inorg Biochem. 2007. PMID: 17723242 Free PMC article.
-
An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins--From superoxide dismutation to H2O2-driven pathways.Redox Biol. 2015 Aug;5:43-65. doi: 10.1016/j.redox.2015.01.017. Epub 2015 Feb 7. Redox Biol. 2015. PMID: 25827425 Free PMC article. Review.
Cited by
-
Ascorbic acid: chemistry, biology and the treatment of cancer.Biochim Biophys Acta. 2012 Dec;1826(2):443-57. doi: 10.1016/j.bbcan.2012.06.003. Epub 2012 Jun 20. Biochim Biophys Acta. 2012. PMID: 22728050 Free PMC article. Review.
-
Redox modulation of HMGB1-related signaling.Antioxid Redox Signal. 2014 Mar 1;20(7):1075-85. doi: 10.1089/ars.2013.5179. Epub 2013 Mar 19. Antioxid Redox Signal. 2014. PMID: 23373897 Free PMC article. Review.
-
A new SOD mimic, Mn(III) ortho N-butoxyethylpyridylporphyrin, combines superb potency and lipophilicity with low toxicity.Free Radic Biol Med. 2012 May 1;52(9):1828-34. doi: 10.1016/j.freeradbiomed.2012.02.006. Epub 2012 Feb 13. Free Radic Biol Med. 2012. PMID: 22336516 Free PMC article.
-
The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells.Redox Biol. 2019 Jan;20:367-378. doi: 10.1016/j.redox.2018.10.016. Epub 2018 Oct 25. Redox Biol. 2019. PMID: 30408752 Free PMC article.
-
Copper(II) Complexes with Carnosine Conjugates of Hyaluronic Acids at Different Dipeptide Loading Percentages Behave as Multiple SOD Mimics and Stimulate Nrf2 Translocation and Antioxidant Response in In Vitro Inflammatory Model.Antioxidants (Basel). 2023 Aug 18;12(8):1632. doi: 10.3390/antiox12081632. Antioxidants (Basel). 2023. PMID: 37627627 Free PMC article.
References
-
- Abashkin YG. Burt SK. (Salen)MnIII compounds as nonpeptidyl mimics of catalase. Mechanism-based tuning of catalase activity: a theoretical study. Inorg Chem. 2005;44:1425–1432. - PubMed
-
- Abidi P. Leers-Sucheta S. Cortez Y. Han J. Azhar S. Evidence that age-related changes in p38 MAP kinase contribute the decreased steroid production by adrenocortical cells from old rats. Aging Cell. 2008;7:168–178. - PubMed
-
- Adam O. Laufs U. Antioxidant effects of statins. Arch Toxicol. 2008;82:885–892. - PubMed
-
- Agadjanian H. Weaver JJ. Mahammed A. Rentsendorj A. Bass S. Kim J. Dmochowski IJ. Margalit R. Gray HB. Gross Z. Medina-Kauwe LK. Specific delivery of corroles to cells via noncovalent conjugates with viral proteins. Pharm Res. 2006;23:367–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

