An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses
- PMID: 20082709
- PMCID: PMC2823673
- DOI: 10.1186/1743-422X-7-9
An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses
Abstract
Background: A growing concern has raised regarding the pandemic potential of the highly pathogenic avian influenza (HPAI) H5N1 viruses. Consequently, there is an urgent need to develop an effective and safe vaccine against the divergent H5N1 influenza viruses. In the present study, we designed a tetra-branched multiple antigenic peptide (MAP)-based vaccine, designated M2e-MAP, which contains the sequence overlapping the highly conserved extracellular domain of matrix protein 2 (M2e) of a HPAI H5N1 virus, and investigated its immune responses and cross-protection against different clades of H5N1 viruses.
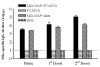
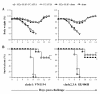
Results: Our results showed that M2e-MAP vaccine induced strong M2e-specific IgG antibody responses following 3-dose immunization of mice with M2e-MAP in the presence of Freunds' or aluminium (alum) adjuvant. M2e-MAP vaccination limited viral replication and attenuated histopathological damage in the challenged mouse lungs. The M2e-MAP-based vaccine protected immunized mice against both clade1: VN/1194 and clade2.3.4: SZ/406H H5N1 virus challenge, being able to counteract weight lost and elevate survival rate following lethal challenge of H5N1 viruses.
Conclusions: These results suggest that M2e-MAP presenting M2e of H5N1 virus has a great potential to be developed into an effective subunit vaccine for the prevention of infection by a broad spectrum of HPAI H5N1 viruses.
Figures
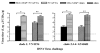


Similar articles
-
An H5N1 M2e-based multiple antigenic peptide vaccine confers heterosubtypic protection from lethal infection with pandemic 2009 H1N1 virus.Virol J. 2010 Jul 12;7:151. doi: 10.1186/1743-422X-7-151. Virol J. 2010. PMID: 20624292 Free PMC article.
-
An M2e-based synthetic peptide vaccine for influenza A virus confers heterosubtypic protection from lethal virus challenge.Virol J. 2013 Jul 9;10:227. doi: 10.1186/1743-422X-10-227. Virol J. 2013. PMID: 23834899 Free PMC article.
-
An H5N1-based matrix protein 2 ectodomain tetrameric peptide vaccine provides cross-protection against lethal infection with H7N9 influenza virus.Emerg Microbes Infect. 2015 Apr;4(4):e22. doi: 10.1038/emi.2015.22. Epub 2015 Apr 8. Emerg Microbes Infect. 2015. PMID: 26038770 Free PMC article.
-
Immunization with M2e-displaying T7 bacteriophage nanoparticles protects against influenza A virus challenge.PLoS One. 2012;7(9):e45765. doi: 10.1371/journal.pone.0045765. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029232 Free PMC article.
-
Influenza A viruses: why focusing on M2e-based universal vaccines.Virus Genes. 2011 Feb;42(1):1-8. doi: 10.1007/s11262-010-0547-7. Epub 2010 Nov 17. Virus Genes. 2011. PMID: 21082230 Review.
Cited by
-
Increased immunogenicity and protective efficacy of influenza M2e fused to a tetramerizing protein.PLoS One. 2012;7(10):e46395. doi: 10.1371/journal.pone.0046395. Epub 2012 Oct 1. PLoS One. 2012. PMID: 23049700 Free PMC article.
-
Differences in the pathogenicity and inflammatory responses induced by avian influenza A/H7N9 virus infection in BALB/c and C57BL/6 mouse models.PLoS One. 2014 Mar 27;9(3):e92987. doi: 10.1371/journal.pone.0092987. eCollection 2014. PLoS One. 2014. PMID: 24676272 Free PMC article.
-
An insight into the epitope-based peptide vaccine design strategy and studies against COVID-19.Turk J Biol. 2020 Jun 21;44(3):215-227. doi: 10.3906/biy-2006-1. eCollection 2020. Turk J Biol. 2020. PMID: 32595358 Free PMC article.
-
DNA-vaccine platform development against H1N1 subtype of swine influenza A viruses.Viral Immunol. 2012 Aug;25(4):297-305. doi: 10.1089/vim.2011.0093. Epub 2012 Jul 20. Viral Immunol. 2012. PMID: 22816869 Free PMC article.
-
Unconjugated Multi-Epitope Peptides Adjuvanted with ALFQ Induce Durable and Broadly Reactive Antibodies to Human and Avian Influenza Viruses.Vaccines (Basel). 2023 Sep 8;11(9):1468. doi: 10.3390/vaccines11091468. Vaccines (Basel). 2023. PMID: 37766144 Free PMC article.
References
-
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

