The emerging role of lysine acetylation of non-nuclear proteins
- PMID: 20082207
- PMCID: PMC11115803
- DOI: 10.1007/s00018-009-0252-7
The emerging role of lysine acetylation of non-nuclear proteins
Abstract
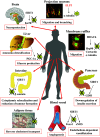
Lysine acetylation is a post-translational modification that critically regulates gene transcription by targeting histones as well as a variety of transcription factors in the nucleus. More recent reports have also demonstrated that numerous proteins located outside the nucleus are also acetylated and that this modification has profound consequences on their functions. This review describes the latest findings on the substrates acetylated outside the nucleus and on the acetylases and deacetylates that catalyse these modifications. Protein acetylation is emerging as a major mechanism by which key proteins are regulated in many physiological processes such as migration, metabolism and aging as well as in pathological circumstances such as cancer and neurodegenerative disorders.
Figures

Similar articles
-
The tale of protein lysine acetylation in the cytoplasm.J Biomed Biotechnol. 2011;2011:970382. doi: 10.1155/2011/970382. Epub 2010 Nov 28. J Biomed Biotechnol. 2011. PMID: 21151618 Free PMC article. Review.
-
The multifaceted role of lysine acetylation in cancer: prognostic biomarker and therapeutic target.Oncotarget. 2016 Aug 23;7(34):55789-55810. doi: 10.18632/oncotarget.10048. Oncotarget. 2016. PMID: 27322556 Free PMC article. Review.
-
The Role of Protein Acetylation in Centrosome Biology.Results Probl Cell Differ. 2019;67:17-25. doi: 10.1007/978-3-030-23173-6_2. Results Probl Cell Differ. 2019. PMID: 31435790 Review.
-
Lysine-specific acetylated proteome from the archaeon Thermococcus gammatolerans reveals the presence of acetylated histones.J Proteomics. 2021 Feb 10;232:104044. doi: 10.1016/j.jprot.2020.104044. Epub 2020 Nov 6. J Proteomics. 2021. PMID: 33161166
-
Lysine acetylation and cancer: A proteomics perspective.J Proteomics. 2017 Jan 6;150:297-309. doi: 10.1016/j.jprot.2016.10.003. Epub 2016 Oct 13. J Proteomics. 2017. PMID: 27746255 Review.
Cited by
-
Differential lysine acetylation profiles of Erwinia amylovora strains revealed by proteomics.J Proteomics. 2013 Feb 21;79:60-71. doi: 10.1016/j.jprot.2012.12.001. Epub 2012 Dec 9. J Proteomics. 2013. PMID: 23234799 Free PMC article.
-
Protein targets of thioacetamide metabolites in rat hepatocytes.Chem Res Toxicol. 2013 Apr 15;26(4):564-74. doi: 10.1021/tx400001x. Epub 2013 Mar 20. Chem Res Toxicol. 2013. PMID: 23465048 Free PMC article.
-
Reversible lysine acetylation regulates activity of human glycine N-acyltransferase-like 2 (hGLYATL2): implications for production of glycine-conjugated signaling molecules.J Biol Chem. 2012 May 11;287(20):16158-67. doi: 10.1074/jbc.M112.347260. Epub 2012 Mar 9. J Biol Chem. 2012. PMID: 22408254 Free PMC article.
-
Acetylation of the RhoA GEF Net1A controls its subcellular localization and activity.J Cell Sci. 2015 Mar 1;128(5):913-22. doi: 10.1242/jcs.158121. Epub 2015 Jan 14. J Cell Sci. 2015. PMID: 25588829 Free PMC article.
-
The tale of protein lysine acetylation in the cytoplasm.J Biomed Biotechnol. 2011;2011:970382. doi: 10.1155/2011/970382. Epub 2010 Nov 28. J Biomed Biotechnol. 2011. PMID: 21151618 Free PMC article. Review.
References
-
- Pazin MJ, Kadonaga JT. What’s up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. - PubMed
-
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. - PubMed
-
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. - PubMed
-
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

