Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection
- PMID: 20081054
- PMCID: PMC2993086
- DOI: 10.1165/rcmb.2009-0244OC
Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection
Abstract
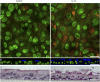
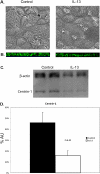
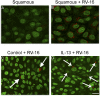
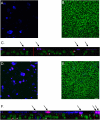
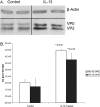
Infection of airway epithelium by rhinovirus is the most common cause of asthma exacerbations. Even in mild asthma, airway epithelium exhibits mucous metaplasia, which increases with increasing severity of the disease. We previously showed that squamous cultures of human airway epithelium manifest rhinoviral infection at levels many times higher than in well-differentiated cultures of a mucociliary phenotype. Here we tested the hypothesis that mucous metaplasia is also associated with increased levels of rhinoviral infection. Mucous metaplasia was induced with IL-13, which doubled the numbers of goblet cells. In both control (mucociliary) and IL-13- treated (mucous metaplastic) cultures, goblet cells were preferentially infected by rhinovirus. IL-13 doubled the numbers of infected cells by increasing the numbers of infected goblet cells. Furthermore, IL-13 increased both the maturity of goblet cells and the probability that a goblet cell would be infected. The infection of cells other than goblet cells was unaltered by IL-13. Treatment with IL-13 did not alter the levels of rhinovirus receptor ICAM-1, nor did the proliferative effects of IL-13 enhance infection, because rhinovirus did not colocalize with dividing cells. However, the induction of mucous metaplasia caused changes in the apical membrane structure, notably a marked decrease in overall ciliation, and an increase in the overall flatness of the apical surface. We conclude that mucous metaplasia in asthma increases the susceptibility of airway epithelium to infection by rhinovirus because of changes in the overall architecture of the apical surface.
Figures



Similar articles
-
Th2-type cytokine-induced mucus metaplasia decreases susceptibility of human bronchial epithelium to rhinovirus infection.Am J Respir Cell Mol Biol. 2014 Aug;51(2):229-41. doi: 10.1165/rcmb.2013-0395OC. Am J Respir Cell Mol Biol. 2014. PMID: 24588727
-
The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice.J Immunol. 2017 Aug 15;199(4):1308-1318. doi: 10.4049/jimmunol.1700216. Epub 2017 Jul 12. J Immunol. 2017. PMID: 28701507 Free PMC article.
-
IFN-γ Blocks Development of an Asthma Phenotype in Rhinovirus-Infected Baby Mice by Inhibiting Type 2 Innate Lymphoid Cells.Am J Respir Cell Mol Biol. 2017 Feb;56(2):242-251. doi: 10.1165/rcmb.2016-0056OC. Am J Respir Cell Mol Biol. 2017. PMID: 27679954 Free PMC article.
-
Rhinovirus Attributes that Contribute to Asthma Development.Immunol Allergy Clin North Am. 2019 Aug;39(3):345-359. doi: 10.1016/j.iac.2019.03.004. Epub 2019 May 7. Immunol Allergy Clin North Am. 2019. PMID: 31284925 Free PMC article. Review.
-
Rhinovirus-Induced Modulation of Epithelial Phenotype: Role in Asthma.Viruses. 2020 Nov 19;12(11):1328. doi: 10.3390/v12111328. Viruses. 2020. PMID: 33227953 Free PMC article. Review.
Cited by
-
The type 2 asthma mediator IL-13 inhibits SARS-CoV-2 infection of bronchial epithelium.bioRxiv [Preprint]. 2021 Feb 25:2021.02.25.432762. doi: 10.1101/2021.02.25.432762. bioRxiv. 2021. Update in: Am J Respir Cell Mol Biol. 2022 Apr;66(4):391-401. doi: 10.1165/rcmb.2021-0364OC. PMID: 33655249 Free PMC article. Updated. Preprint.
-
Goblet Cell Hyperplasia Requires High Bicarbonate Transport To Support Mucin Release.Sci Rep. 2016 Oct 27;6:36016. doi: 10.1038/srep36016. Sci Rep. 2016. PMID: 27786259 Free PMC article.
-
Macrophages Orchestrate Airway Inflammation, Remodeling, and Resolution in Asthma.Int J Mol Sci. 2023 Jun 21;24(13):10451. doi: 10.3390/ijms241310451. Int J Mol Sci. 2023. PMID: 37445635 Free PMC article. Review.
-
Polarization of protease-activated receptor 2 (PAR-2) signaling is altered during airway epithelial remodeling and deciliation.J Biol Chem. 2020 May 8;295(19):6721-6740. doi: 10.1074/jbc.RA120.012710. Epub 2020 Apr 2. J Biol Chem. 2020. PMID: 32241907 Free PMC article.
-
Allergic sensitization frequency and wheezing differences in early life between black and white children.Allergy Asthma Proc. 2012 Nov-Dec;33(6):493-9. doi: 10.2500/aap.2012.33.3600. Allergy Asthma Proc. 2012. PMID: 23394507 Free PMC article.
References
-
- van Elden LJR, Sachs APE, van Loon AM, Haarman M, van de Vijver DA, Kimman TG, Zuithoff P, Schipper PJ, Verheij TJM, Nijhuis M. Enhanced severity of virus associated lower respiratory tract disease in asthma patients may not be associated with delayed viral clearance and increased viral load in the upper respiratory tract. J Clin Virol 41(2):116–121. - PMC - PubMed
-
- Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med 2005;11:21–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

