Vaccination of mice with salmonella expressing VapA: mucosal and systemic Th1 responses provide protection against Rhodococcus equi infection
- PMID: 20072623
- PMCID: PMC2800180
- DOI: 10.1371/journal.pone.0008644
Vaccination of mice with salmonella expressing VapA: mucosal and systemic Th1 responses provide protection against Rhodococcus equi infection
Abstract
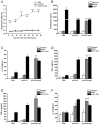
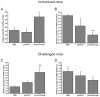
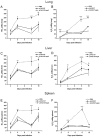
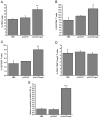
Conventional vaccines to prevent the pneumonia caused by Rhodococcus equi have not been successful. We have recently demonstrated that immunization with Salmonella enterica Typhimurium expressing the VapA antigen protects mice against R. equi infection. We now report that oral vaccination of mice with this recombinant strain results in high and persistent fecal levels of antigen-specific IgA, and specific proliferation of the spleen cells of immunized mice in response to the in vitro stimulation with R. equi antigen. After in vitro stimulation, spleen cells of immunized mice produce high levels of Th1 cytokines and show a prominent mRNA expression of the Th1 transcription factor T-bet, in detriment of the Th2 transcription factor GATA-3. Following R. equi challenge, a high H2O2, NO, IL-12, and IFN-gamma content is detected in the organs of immunized mice. On the other hand, TNF-alpha and IL-4 levels are markedly lower in the organs of vaccinated mice, compared with the non-vaccinated ones. The IL-10 content and the mRNA transcription level of TGF-beta are also higher in the organs of immunized mice. A greater incidence of CD4+ and CD8+ T cells and B lymphocytes is verified in vaccinated mice. However, there is no difference between vaccinated and non-vaccinated mice in terms of the frequency of CD4+CD25+Foxp3+ T cells. Finally, we show that the vaccination confers a long-term protection against R. equi infection. Altogether, these data indicate that the oral vaccination of mice with S. enterica Typhimurium expressing VapA induces specific and long-lasting humoral and cellular responses against the pathogen, which are appropriately regulated and allow tissue integrity after challenge.
Conflict of interest statement
Figures
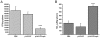
Similar articles
-
Nasal vaccination with attenuated Salmonella expressing VapA: TLR2 activation is not essential for protection against R. equi infection.Vaccine. 2013 Sep 23;31(41):4528-35. doi: 10.1016/j.vaccine.2013.07.067. Epub 2013 Aug 7. Vaccine. 2013. PMID: 23933366
-
Analysis of anamnestic immune responses in adult horses and priming in neonates induced by a DNA vaccine expressing the vapA gene of Rhodococcus equi.Vaccine. 2003 Sep 8;21(25-26):3815-25. doi: 10.1016/s0264-410x(03)00329-3. Vaccine. 2003. PMID: 12922115
-
Oral administration of a live attenuated Salmonella vaccine strain expressing the VapA protein induces protection against infection by Rhodococcus equi.Microbes Infect. 2007 Mar;9(3):382-90. doi: 10.1016/j.micinf.2006.12.019. Epub 2007 Jan 12. Microbes Infect. 2007. PMID: 17307012
-
Current understanding of the equine immune response to Rhodococcus equi. An immunological review of R. equi pneumonia.Vet Immunol Immunopathol. 2010 May 15;135(1-2):1-11. doi: 10.1016/j.vetimm.2009.12.004. Epub 2009 Dec 23. Vet Immunol Immunopathol. 2010. PMID: 20064668 Review.
-
Immunity to Rhodococcus equi.Vet Microbiol. 1997 Jun 16;56(3-4):177-85. doi: 10.1016/s0378-1135(97)00086-2. Vet Microbiol. 1997. PMID: 9226832 Review.
Cited by
-
The steroid catabolic pathway of the intracellular pathogen Rhodococcus equi is important for pathogenesis and a target for vaccine development.PLoS Pathog. 2011 Aug;7(8):e1002181. doi: 10.1371/journal.ppat.1002181. Epub 2011 Aug 25. PLoS Pathog. 2011. PMID: 21901092 Free PMC article.
-
The type of anticoagulant used for plasma collection affects in vitro Rhodococcus equi assays.BMC Res Notes. 2022 Feb 14;15(1):50. doi: 10.1186/s13104-022-05933-4. BMC Res Notes. 2022. PMID: 35164828 Free PMC article.
-
Attenuated Salmonella typhimurium SV4089 as a potential carrier of oral DNA vaccine in chickens.J Biomed Biotechnol. 2012;2012:264986. doi: 10.1155/2012/264986. Epub 2012 Jun 4. J Biomed Biotechnol. 2012. PMID: 22701301 Free PMC article.
-
Evaluation of vaccine candidates against Rhodococcus equi in BALB/c mice infection model: cellular and humoral immune responses.BMC Microbiol. 2024 Jul 8;24(1):249. doi: 10.1186/s12866-024-03408-z. BMC Microbiol. 2024. PMID: 38977999 Free PMC article.
-
Vaccination of Mice with Virulence-Associated Protein G (VapG) Antigen Confers Partial Protection against Rhodococcus equi Infection through Induced Humoral Immunity.Front Microbiol. 2017 May 11;8:857. doi: 10.3389/fmicb.2017.00857. eCollection 2017. Front Microbiol. 2017. PMID: 28553279 Free PMC article.
References
-
- Giguere S, Prescott JF. Clinical manifestations, diagnosis, treatment, and prevention of Rhodococcus equi infections in foals. Vet Microbiol. 1997;56:313–334. - PubMed
-
- Donisi A, Suardi MG, Casari S, Longo M, Cadeo GP, et al. Rhodococcus equi infection in HIV-infected patients. AIDS. 1996;10:359–362. - PubMed
-
- Meijer WG, Prescott JF. Rhodococcus equi. Vet Res. 2004;35:383–396. - PubMed
-
- Magnusson H. Spezifische infektiose pneumonie beim Fohlen. Ein neuer eiterreger beim Pferd. Arch Wiss Prakt Tierhelkd. 1923;50:22–38.
-
- Hondalus MK. Pathogenesis and virulence of Rhodococcus equi. Vet Microbiol. 1997;56:257–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

