Basic mechanisms of neurodegeneration: a critical update
- PMID: 20070435
- PMCID: PMC3823450
- DOI: 10.1111/j.1582-4934.2010.01010.x
Basic mechanisms of neurodegeneration: a critical update
Abstract
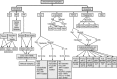
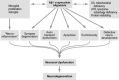
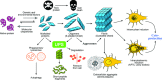
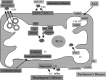
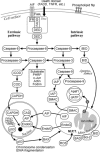
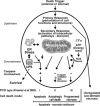
Neurodegenerative diseases are characterized by progressive dysfunction of specific populations of neurons, determining clinical presentation. Neuronal loss is associated with extra and intracellular accumulation of misfolded proteins, the hallmarks of many neurodegenerative proteinopathies. Major basic processes include abnormal protein dynamics due to deficiency of the ubiquitin-proteosome-autophagy system, oxidative stress and free radical formation, mitochondrial dysfunction, impaired bioenergetics, dysfunction of neurotrophins, 'neuroinflammatory' processes and (secondary) disruptions of neuronal Golgi apparatus and axonal transport. These interrelated mechanisms lead to programmed cell death is a long run over many years. Neurodegenerative disorders are classified according to known genetic mechanisms or to major components of protein deposits, but recent studies showed both overlap and intraindividual diversities between different phenotypes. Synergistic mechanisms between pathological proteins suggest common pathogenic mechanisms. Animal models and other studies have provided insight into the basic neurodegeneration and cell death programs, offering new ways for future prevention/treatment strategies.
Figures
Similar articles
-
Recent advances in our understanding of neurodegeneration.J Neural Transm (Vienna). 2009 Sep;116(9):1111-62. doi: 10.1007/s00702-009-0240-y. Epub 2009 Jun 5. J Neural Transm (Vienna). 2009. PMID: 19707851 Review.
-
General aspects of neurodegeneration.J Neural Transm Suppl. 2003;(65):101-44. doi: 10.1007/978-3-7091-0643-3_7. J Neural Transm Suppl. 2003. PMID: 12946052 Review.
-
Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities.Cell Death Differ. 2021 Feb;28(2):570-590. doi: 10.1038/s41418-020-00706-7. Epub 2021 Jan 7. Cell Death Differ. 2021. PMID: 33414510 Free PMC article. Review.
-
Autophagy and neurodegeneration: when the cleaning crew goes on strike.Lancet Neurol. 2007 Apr;6(4):352-61. doi: 10.1016/S1474-4422(07)70076-5. Lancet Neurol. 2007. PMID: 17362839 Review.
-
Autophagy and apoptosis dysfunction in neurodegenerative disorders.Prog Neurobiol. 2014 Jan;112:24-49. doi: 10.1016/j.pneurobio.2013.10.004. Epub 2013 Nov 6. Prog Neurobiol. 2014. PMID: 24211851 Review.
Cited by
-
A missense change in the ATG4D gene links aberrant autophagy to a neurodegenerative vacuolar storage disease.PLoS Genet. 2015 Apr 15;11(4):e1005169. doi: 10.1371/journal.pgen.1005169. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25875846 Free PMC article.
-
Citrus Polyphenols in Brain Health and Disease: Current Perspectives.Front Neurosci. 2021 Feb 19;15:640648. doi: 10.3389/fnins.2021.640648. eCollection 2021. Front Neurosci. 2021. PMID: 33679318 Free PMC article. Review.
-
Exploring the Role of Reactive Oxygen Species in the Pathogenesis and Pathophysiology of Alzheimer's and Parkinson's Disease and the Efficacy of Antioxidant Treatment.Antioxidants (Basel). 2024 Sep 20;13(9):1138. doi: 10.3390/antiox13091138. Antioxidants (Basel). 2024. PMID: 39334797 Free PMC article. Review.
-
Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration.Front Integr Neurosci. 2022 Jan 17;15:747901. doi: 10.3389/fnint.2021.747901. eCollection 2021. Front Integr Neurosci. 2022. PMID: 35111001 Free PMC article. Review.
-
Metabotropic glutamate receptor subtype 5 is altered in LPS-induced murine neuroinflammation model and in the brains of AD and ALS patients.Eur J Nucl Med Mol Imaging. 2019 Feb;46(2):407-420. doi: 10.1007/s00259-018-4179-9. Epub 2018 Oct 5. Eur J Nucl Med Mol Imaging. 2019. PMID: 30291374
References
-
- Jellinger KA. Recent advances in our understanding of neurodegeneration. J Neural Transm. 2009;116:1111–62. - PubMed
-
- Skovronsky DM, Lee VM-Y, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol Mech Dis. 2006;1:151–70. - PubMed
-
- Uversky VN. Intrinsic disorder in proteins associated with neurodegenerative diseases. Front Biosci. 2009;14:5188–238. - PubMed
-
- Herczenik E, Gebbink MF. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 2008;22:2115–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

