Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death
- PMID: 20067982
- PMCID: PMC2818107
- DOI: 10.1093/jac/dkp486
Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death
Abstract
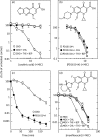
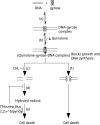
Background: Quinolone-mediated death of Escherichia coli has been proposed to occur by two pathways. One is blocked by inhibitors of protein synthesis; the other is not. It is currently unknown how these two pathways fit with the recent observation that hydroxyl radical accumulation is associated with quinolone lethality.
Methods: E. coli was treated with thiourea plus 2,2'-bipyridyl to block hydroxyl radical accumulation, and the effect on quinolone lethality was measured for quinolones that distinguished the two lethal pathways: oxolinic acid requires protein synthesis to kill E. coli, while PD161144, a C-8-methoxy fluoroquinolone, does not. The lethal activity of another fluoroquinolone, moxifloxacin, was partially blocked by the presence of chloramphenicol, an inhibitor of protein synthesis. That feature made it possible to determine whether the effects of chloramphenicol and thiourea plus 2,2'-bipyridyl were additive.
Results: Lethal activity of oxolinic acid was completely blocked by thiourea plus 2,2'-bipyridyl and by chloramphenicol. In contrast, PD161144 lethality was unaffected by these treatments. With moxifloxacin, both chloramphenicol and thiourea plus 2,2'-bipyridyl separately exhibited the same partial inhibition of quinolone lethality. No additivity in protection from moxifloxacin lethality was observed when thiourea, 2,2'-bipyridyl and chloramphenicol were combined and compared with the effect of chloramphenicol or thiourea plus 2,2'-bipyridyl used separately.
Conclusions: Inhibitor studies indicated that hydroxyl radical action contributes to quinolone-mediated cell death occurring via the chloramphenicol-sensitive lethal pathway but not via the chloramphenicol-insensitive pathway.
Figures
Similar articles
-
Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents.Antimicrob Agents Chemother. 2012 Nov;56(11):6048-50. doi: 10.1128/AAC.00754-12. Epub 2012 Sep 4. Antimicrob Agents Chemother. 2012. PMID: 22948880 Free PMC article.
-
Effect of anaerobic growth on quinolone lethality with Escherichia coli.Antimicrob Agents Chemother. 2007 Jan;51(1):28-34. doi: 10.1128/AAC.00739-06. Epub 2006 Oct 16. Antimicrob Agents Chemother. 2007. PMID: 17043118 Free PMC article.
-
Reactive oxygen species play a dominant role in all pathways of rapid quinolone-mediated killing.J Antimicrob Chemother. 2020 Mar 1;75(3):576-585. doi: 10.1093/jac/dkz485. J Antimicrob Chemother. 2020. PMID: 31793990
-
Moxifloxacin: a respiratory fluoroquinolone.Expert Opin Pharmacother. 2008 Jul;9(10):1755-72. doi: 10.1517/14656566.9.10.1755. Expert Opin Pharmacother. 2008. PMID: 18570608 Review.
-
Bacterial death from treatment with fluoroquinolones and other lethal stressors.Expert Rev Anti Infect Ther. 2021 May;19(5):601-618. doi: 10.1080/14787210.2021.1840353. Epub 2020 Nov 16. Expert Rev Anti Infect Ther. 2021. PMID: 33081547 Review.
Cited by
-
Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells.Sci Transl Med. 2013 Jul 3;5(192):192ra85. doi: 10.1126/scitranslmed.3006055. Sci Transl Med. 2013. PMID: 23825301 Free PMC article.
-
A ferritin mutant of Mycobacterium tuberculosis is highly susceptible to killing by antibiotics and is unable to establish a chronic infection in mice.Infect Immun. 2012 Oct;80(10):3650-9. doi: 10.1128/IAI.00229-12. Epub 2012 Jul 16. Infect Immun. 2012. PMID: 22802345 Free PMC article.
-
Perturbation of iron homeostasis promotes the evolution of antibiotic resistance.Mol Biol Evol. 2014 Oct;31(10):2793-804. doi: 10.1093/molbev/msu223. Epub 2014 Jul 24. Mol Biol Evol. 2014. PMID: 25063442 Free PMC article.
-
Effects of antibiotics (enrofloxacin) on microbial community of water and sediment in an aquatic ecological model.Front Vet Sci. 2023 May 30;10:1151988. doi: 10.3389/fvets.2023.1151988. eCollection 2023. Front Vet Sci. 2023. PMID: 37323836 Free PMC article.
-
Absence of tmRNA Has a Protective Effect against Fluoroquinolones in Streptococcus pneumoniae.Front Microbiol. 2017 Jan 10;7:2164. doi: 10.3389/fmicb.2016.02164. eCollection 2016. Front Microbiol. 2017. PMID: 28119681 Free PMC article.
References
-
- Chen C-R, Malik M, Snyder M, et al. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–37. - PubMed
-
- Malik M, Zhao X, Drlica K. Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones. Mol Microbiol. 2006;61:810–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

