Cell adhesion, the backbone of the synapse: "vertebrate" and "invertebrate" perspectives
- PMID: 20066100
- PMCID: PMC2773626
- DOI: 10.1101/cshperspect.a003079
Cell adhesion, the backbone of the synapse: "vertebrate" and "invertebrate" perspectives
Abstract
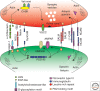
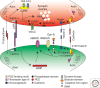
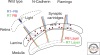
Synapses are asymmetric intercellular junctions that mediate neuronal communication. The number, type, and connectivity patterns of synapses determine the formation, maintenance, and function of neural circuitries. The complexity and specificity of synaptogenesis relies upon modulation of adhesive properties, which regulate contact initiation, synapse formation, maturation, and functional plasticity. Disruption of adhesion may result in structural and functional imbalance that may lead to neurodevelopmental diseases, such as autism, or neurodegeneration, such as Alzheimer's disease. Therefore, understanding the roles of different adhesion protein families in synapse formation is crucial for unraveling the biology of neuronal circuit formation, as well as the pathogenesis of some brain disorders. The present review summarizes some of the knowledge that has been acquired in vertebrate and invertebrate genetic model organisms.
Figures

Similar articles
-
Cell adhesion molecules at the synapse.Front Biosci. 2006 Sep 1;11:2400-19. doi: 10.2741/1978. Front Biosci. 2006. PMID: 16720322 Review.
-
Mechanisms of vertebrate synaptogenesis.Annu Rev Neurosci. 2005;28:251-74. doi: 10.1146/annurev.neuro.27.070203.144336. Annu Rev Neurosci. 2005. PMID: 16022596 Review.
-
SynCAMs extend their functions beyond the synapse.Eur J Neurosci. 2014 Jun;39(11):1752-60. doi: 10.1111/ejn.12544. Epub 2014 Mar 15. Eur J Neurosci. 2014. PMID: 24628990 Review.
-
Synapse formation in developing neural circuits.Curr Top Dev Biol. 2009;87:53-79. doi: 10.1016/S0070-2153(09)01202-2. Curr Top Dev Biol. 2009. PMID: 19427516 Free PMC article. Review.
-
Cadherins and synaptic specificity.J Neurosci Res. 1999 Oct 1;58(1):130-8. J Neurosci Res. 1999. PMID: 10491578 Review.
Cited by
-
Conservation and Innovation: Versatile Roles for LRP4 in Nervous System Development.J Dev Biol. 2021 Mar 14;9(1):9. doi: 10.3390/jdb9010009. J Dev Biol. 2021. PMID: 33799485 Free PMC article. Review.
-
Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95.Cell. 2012 Dec 21;151(7):1581-94. doi: 10.1016/j.cell.2012.11.040. Cell. 2012. PMID: 23260144 Free PMC article.
-
Lateral assembly of the immunoglobulin protein SynCAM 1 controls its adhesive function and instructs synapse formation.EMBO J. 2011 Sep 16;30(23):4728-38. doi: 10.1038/emboj.2011.336. EMBO J. 2011. PMID: 21926970 Free PMC article.
-
LAR-RPTP Clustering Is Modulated by Competitive Binding between Synaptic Adhesion Partners and Heparan Sulfate.Front Mol Neurosci. 2017 Oct 13;10:327. doi: 10.3389/fnmol.2017.00327. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29081732 Free PMC article.
-
Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss.Nat Neurosci. 2013 Jun;16(6):706-13. doi: 10.1038/nn.3395. Epub 2013 May 5. Nat Neurosci. 2013. PMID: 23644483
References
-
- Abe K, Chisaka O, Van Roy F, Takeichi M 2004. Stability of dendritic spines and synaptic contacts is controlled by α N-catenin. Nat Neurosci 7:357–363 - PubMed
-
- Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholm AD, Jin Y 2005. The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J Neurosci 25:7517–7528 - PMC - PubMed
-
- Artero RD, Castanon I, Baylies MK 2001. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development 128:4251–4264 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
