Activation of mutant enzyme function in vivo by proteasome inhibitors and treatments that induce Hsp70
- PMID: 20066033
- PMCID: PMC2795852
- DOI: 10.1371/journal.pgen.1000807
Activation of mutant enzyme function in vivo by proteasome inhibitors and treatments that induce Hsp70
Abstract
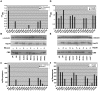
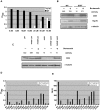
Missense mutant proteins, such as those produced in individuals with genetic diseases, are often misfolded and subject to processing by intracellular quality control systems. Previously, we have shown using a yeast system that enzymatic function could be restored to I278T cystathionine beta-synthase (CBS), a cause of homocystinuria, by treatments that affect the intracellular chaperone environment. Here, we extend these studies and show that it is possible to restore significant levels of enzyme activity to 17 of 18 (94%) disease causing missense mutations in human cystathionine beta-synthase (CBS) expressed in Saccharomyces cerevisiae by exposure to ethanol, proteasome inhibitors, or deletion of the Hsp26 small heat shock protein. All three of these treatments induce Hsp70, which is necessary but not sufficient for rescue. In addition to CBS, these same treatments can rescue disease-causing mutations in human p53 and the methylene tetrahydrofolate reductase gene. These findings do not appear restricted to S. cerevisiae, as proteasome inhibitors can restore significant CBS enzymatic activity to CBS alleles expressed in fibroblasts derived from homocystinuric patients and in a mouse model for homocystinuria that expresses human I278T CBS. These findings suggest that proteasome inhibitors and other Hsp70 inducing agents may be useful in the treatment of a variety of genetic diseases caused by missense mutations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Genetic and Pharmacological Modulation of Cellular Proteostasis Leads to Partial Functional Rescue of Homocystinuria-Causing Cystathionine-Beta Synthase Variants.Mol Cell Biol. 2023;43(12):664-674. doi: 10.1080/10985549.2023.2284147. Epub 2023 Dec 20. Mol Cell Biol. 2023. PMID: 38051092 Free PMC article.
-
Correction of cystathionine β-synthase deficiency in mice by treatment with proteasome inhibitors.Hum Mutat. 2013 Aug;34(8):1085-93. doi: 10.1002/humu.22335. Epub 2013 May 13. Hum Mutat. 2013. PMID: 23592311 Free PMC article.
-
Functional rescue of mutant human cystathionine beta-synthase by manipulation of Hsp26 and Hsp70 levels in Saccharomyces cerevisiae.J Biol Chem. 2009 Feb 13;284(7):4238-45. doi: 10.1074/jbc.M806387200. Epub 2008 Dec 12. J Biol Chem. 2009. PMID: 19074437 Free PMC article.
-
Cystathionine beta-synthase mutations in homocystinuria.Hum Mutat. 1999;13(5):362-75. doi: 10.1002/(SICI)1098-1004(1999)13:5<362::AID-HUMU4>3.0.CO;2-K. Hum Mutat. 1999. PMID: 10338090 Review.
-
Recent therapeutic approaches to cystathionine beta-synthase-deficient homocystinuria.Br J Pharmacol. 2023 Feb;180(3):264-278. doi: 10.1111/bph.15991. Epub 2022 Dec 8. Br J Pharmacol. 2023. PMID: 36417581 Free PMC article. Review.
Cited by
-
Examination of two different proteasome inhibitors in reactivating mutant human cystathionine β-synthase in mice.PLoS One. 2023 Jun 15;18(6):e0286550. doi: 10.1371/journal.pone.0286550. eCollection 2023. PLoS One. 2023. PMID: 37319242 Free PMC article.
-
Surrogate genetics and metabolic profiling for characterization of human disease alleles.Genetics. 2012 Apr;190(4):1309-23. doi: 10.1534/genetics.111.137471. Epub 2012 Jan 20. Genetics. 2012. PMID: 22267502 Free PMC article.
-
Genetic and Pharmacological Modulation of Cellular Proteostasis Leads to Partial Functional Rescue of Homocystinuria-Causing Cystathionine-Beta Synthase Variants.Mol Cell Biol. 2023;43(12):664-674. doi: 10.1080/10985549.2023.2284147. Epub 2023 Dec 20. Mol Cell Biol. 2023. PMID: 38051092 Free PMC article.
-
How to fix a broken protein: restoring function to mutant human cystathionine β-synthase.Hum Genet. 2022 Jul;141(7):1299-1308. doi: 10.1007/s00439-021-02386-w. Epub 2021 Oct 12. Hum Genet. 2022. PMID: 34636997 Free PMC article. Review.
-
The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer.Antioxid Redox Signal. 2015 Feb 10;22(5):424-48. doi: 10.1089/ars.2014.5933. Epub 2014 Jun 20. Antioxid Redox Signal. 2015. PMID: 24730679 Free PMC article. Review.
References
-
- Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003;21:577–581. - PubMed
-
- Bross P, Corydon TJ, Andresen BS, Jorgensen MM, Bolund L, et al. Protein misfolding and degradation in genetic diseases. Hum Mutat. 1999;14:186–198. - PubMed
-
- Kruger WD, Wang L, Jhee KH, Singh RH, Elsas LJ,, 2nd Cystathionine beta-synthase deficiency in Georgia (USA): correlation of clinical and biochemical phenotype with genotype. Hum Mutat. 2003;22:434–441. - PubMed
-
- Wilcken DE, Wilcken B. The natural history of vascular disease in homocystinuria and the effects of treatment. Journal of Inherited Metabolic Disease. 1997;20:295–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

