Estrogen receptors recruit SMRT and N-CoR corepressors through newly recognized contacts between the corepressor N terminus and the receptor DNA binding domain
- PMID: 20065040
- PMCID: PMC2832498
- DOI: 10.1128/MCB.01002-09
Estrogen receptors recruit SMRT and N-CoR corepressors through newly recognized contacts between the corepressor N terminus and the receptor DNA binding domain
Abstract
Estrogen receptors (ERs) are hormone-regulated transcription factors that regulate key aspects of reproduction and development. ERs are unusual in that they do not typically repress transcription in the absence of hormone but instead possess otherwise cryptic repressive functions that are revealed upon binding to certain hormone antagonists. The roles of corepressors in the control of these aspects of ER function are complex and incompletely understood. We report here that ERs recruit SMRT through an unusual mode of interaction involving multiple contact surfaces. Two surfaces of SMRT, located at the N- and C-terminal domains, contribute to the recruitment of the corepressor to ERs in vitro and are crucial for the corepressor modulation of ER transcriptional activity in cells. These corepressor surfaces contact the DNA binding domain of the receptor, rather than the hormone binding domain previously elucidated for other corepressor/nuclear receptor interactions, and are modulated by the ER's recognition of cognate DNA binding sites. Several additional nuclear receptors, and at least one other corepressor, N-CoR, share aspects of this novel mode of corepressor recruitment. Our results highlight a molecular mechanism that helps explain several previously paradoxical aspects of ER-mediated transcriptional antagonism, which may have a broader significance for an understanding of target gene repression by other nuclear receptors.
Figures
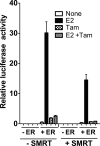
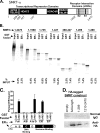
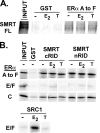
 100%). The means and standard errors for three experiments are shown. All assays were performed in the absence of hormone ligand. (D) Coimmunoprecipitation of SMRT proteins and ERα from cells. Hemagglutinin (HA)-SMRTα, HA-SMRTα(1-289), HA-SMRTα(1210-2470), or the empty HA tag expression vector (HA) was transfected into HeLa cells together with an ERα expression vector. The cells were lysed, the lysates were immunoprecipitated with either nonimmune (“Normal IgG”) or anti-HA antibodies, and the immunoprecipitates were resolved by SDS-PAGE and were visualized using anti-ERα sera.
100%). The means and standard errors for three experiments are shown. All assays were performed in the absence of hormone ligand. (D) Coimmunoprecipitation of SMRT proteins and ERα from cells. Hemagglutinin (HA)-SMRTα, HA-SMRTα(1-289), HA-SMRTα(1210-2470), or the empty HA tag expression vector (HA) was transfected into HeLa cells together with an ERα expression vector. The cells were lysed, the lysates were immunoprecipitated with either nonimmune (“Normal IgG”) or anti-HA antibodies, and the immunoprecipitates were resolved by SDS-PAGE and were visualized using anti-ERα sera.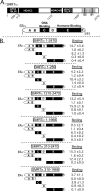
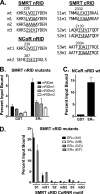

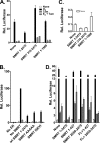
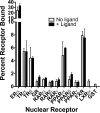
 100%). The means and standard deviations for two experiments are shown.
100%). The means and standard deviations for two experiments are shown.Similar articles
-
Characterization of receptor interaction and transcriptional repression by the corepressor SMRT.Mol Endocrinol. 1997 Dec;11(13):2025-37. doi: 10.1210/mend.11.13.0028. Mol Endocrinol. 1997. PMID: 9415406
-
Corepressor SMRT functions as a coactivator for thyroid hormone receptor T3Ralpha from a negative hormone response element.J Biol Chem. 2002 Dec 20;277(51):49517-22. doi: 10.1074/jbc.M209546200. Epub 2002 Oct 17. J Biol Chem. 2002. PMID: 12388540
-
Gene silencing by chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is mediated by transcriptional corepressors, nuclear receptor-corepressor (N-CoR) and silencing mediator for retinoic acid receptor and thyroid hormone receptor (SMRT).Mol Endocrinol. 1997 Jun;11(6):714-24. doi: 10.1210/mend.11.6.0002. Mol Endocrinol. 1997. PMID: 9171235
-
N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors.Curr Top Microbiol Immunol. 2003;274:237-68. doi: 10.1007/978-3-642-55747-7_9. Curr Top Microbiol Immunol. 2003. PMID: 12596910 Review.
-
Corepressor recruitment by agonist-bound nuclear receptors.Vitam Horm. 2004;68:123-43. doi: 10.1016/S0083-6729(04)68004-6. Vitam Horm. 2004. PMID: 15193453 Review.
Cited by
-
The Ddx5 and Ddx17 RNA helicases are cornerstones in the complex regulatory array of steroid hormone-signaling pathways.Nucleic Acids Res. 2014 Feb;42(4):2197-207. doi: 10.1093/nar/gkt1216. Epub 2013 Nov 25. Nucleic Acids Res. 2014. PMID: 24275493 Free PMC article.
-
Nuclear receptor coregulators as a new paradigm for therapeutic targeting.Adv Drug Deliv Rev. 2010 Oct 30;62(13):1227-37. doi: 10.1016/j.addr.2010.09.016. Epub 2010 Oct 7. Adv Drug Deliv Rev. 2010. PMID: 20933027 Free PMC article. Review.
-
Targeting BIG3-PHB2 interaction to overcome tamoxifen resistance in breast cancer cells.Nat Commun. 2013;4:2443. doi: 10.1038/ncomms3443. Nat Commun. 2013. PMID: 24051437 Free PMC article.
-
Antiestrogens: structure-activity relationships and use in breast cancer treatment.J Mol Endocrinol. 2017 Jan;58(1):R15-R31. doi: 10.1530/JME-16-0024. Epub 2016 Oct 11. J Mol Endocrinol. 2017. PMID: 27729460 Free PMC article. Review.
-
miR-10a/b-5p-NCOR2 Regulates Insulin-Resistant Diabetes in Female Mice.Int J Mol Sci. 2024 Sep 21;25(18):10147. doi: 10.3390/ijms251810147. Int J Mol Sci. 2024. PMID: 39337631 Free PMC article.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Wiley Interscience, New York, NY.
-
- Brzozowski, A. M., A. C. W. Pike, Z. Dauter, R. E. Hubbard, T. Bonn, O. Engstrom, L. Ohman, G. L. Greene, J. A. Gustafsson, and M. Carlquist. 1997. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753-758. - PubMed
-
- Chambon, P. 1996. A decade of molecular biology of retinoic acid receptors. FASEB J. 10:940-954. - PubMed
-
- Chatterjee, V. K. 2008. Nuclear receptors and human disease: resistance to thyroid hormone and lipodystrophic insulin resistance. Ann. Endocrinol. (Paris) 69:103-106. - PubMed
-
- Chen, J. D., and R. M. Evans. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454-457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
