Evaluation of 5-ethynyl-2'-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system
- PMID: 20064490
- PMCID: PMC2826567
- DOI: 10.1016/j.brainres.2009.12.092
Evaluation of 5-ethynyl-2'-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system
Abstract
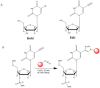
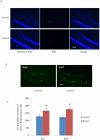
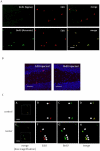
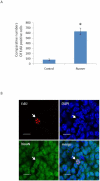
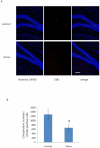
Recently, a novel method for detection of DNA synthesis has been developed based on the incorporation of 5-ethynyl-2'-deoxyuridine (EdU), a thymidine analogue, into cellular DNA and the subsequent reaction of EdU with a fluorescent azide in a copper-catalyzed [3+2] cycloaddition ("Click" reaction). In the present study, we evaluated this method for studying cell proliferation in the adult central nervous system in comparison with the "gold standard" method of 5-bromo-2'-deoxyuridine (BrdU) staining using two behavioral paradigms, voluntary exercise and restraint stress. Our data demonstrate that the number of EdU-positive cells in the dentate gyrus of the hippocampus (DG) slightly increased in an EdU dose-dependent manner in both the control and voluntary exercise (running) mouse groups. The number of EdU-labeled cells was comparable to the number of BrdU-labeled cells in both the control and running mice. Furthermore, EdU and BrdU co-localized to the same cells within the DG. Voluntary exercise significantly increased the number of EdU- and BrdU-positive cells in the DG. In contrast, restraint stress significantly decreased the number of EdU-positive cells. The EdU-positive cells differentiated into mature neurons. EdU staining is compatible with immunohistochemical staining of other antigens. Moreover, our data demonstrated EdU staining can be combined with BrdU staining, providing a valuable tool of double labeling DNA synthesis, e.g., for tracking the two populations of neurons generated at different time points. In conclusion, our results suggest that EdU staining is a fast, sensitive and reproducible method to study cell proliferation in the central nervous system.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Dual-pulse labeling using 5-ethynyl-2'-deoxyuridine (EdU) and 5-bromo-2'-deoxyuridine (BrdU) in flow cytometry.Curr Protoc Cytom. 2011 Jan;Chapter 7:7.38.1-7.38.15. doi: 10.1002/0471142956.cy0738s55. Curr Protoc Cytom. 2011. PMID: 21207361
-
Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice.Hippocampus. 2009 Oct;19(10):889-97. doi: 10.1002/hipo.20514. Hippocampus. 2009. PMID: 18958850
-
The bioavailability time of commonly used thymidine analogues after intraperitoneal delivery in mice: labeling kinetics in vivo and clearance from blood serum.Histochem Cell Biol. 2022 Feb;157(2):239-250. doi: 10.1007/s00418-021-02048-y. Epub 2021 Nov 10. Histochem Cell Biol. 2022. PMID: 34757474 Free PMC article.
-
Thymidine analogues for tracking DNA synthesis.Molecules. 2011 Sep 15;16(9):7980-93. doi: 10.3390/molecules16097980. Molecules. 2011. PMID: 21921870 Free PMC article. Review.
-
Incorporation of 5-Bromo-2'-deoxyuridine into DNA and Proliferative Behavior of Cerebellar Neuroblasts: All That Glitters Is Not Gold.Cells. 2021 Jun 10;10(6):1453. doi: 10.3390/cells10061453. Cells. 2021. PMID: 34200598 Free PMC article. Review.
Cited by
-
A new method for in vitro detection of bromodeoxyuridine in serum: a proof of concept in a songbird species, the canary.PLoS One. 2013 May 14;8(5):e63692. doi: 10.1371/journal.pone.0063692. Print 2013. PLoS One. 2013. PMID: 23691086 Free PMC article.
-
Targeting cardiomyocyte proliferation as a key approach of promoting heart repair after injury.Mol Biomed. 2021 Nov 5;2(1):34. doi: 10.1186/s43556-021-00047-y. Mol Biomed. 2021. PMID: 35006441 Free PMC article. Review.
-
Activation of adult mammalian retinal stem cells in vivo via antagonism of BMP and sFRP2.Stem Cell Res Ther. 2021 Oct 30;12(1):560. doi: 10.1186/s13287-021-02630-0. Stem Cell Res Ther. 2021. PMID: 34717744 Free PMC article.
-
c-kit Haploinsufficiency impairs adult cardiac stem cell growth, myogenicity and myocardial regeneration.Cell Death Dis. 2019 Jun 4;10(6):436. doi: 10.1038/s41419-019-1655-5. Cell Death Dis. 2019. PMID: 31164633 Free PMC article.
-
Stand-up exercise training facilitates muscle recovery from disuse atrophy by stimulating myogenic satellite cell proliferation in mice.Physiol Rep. 2014 Nov 3;2(11):e12185. doi: 10.14814/phy2.12185. Print 2014 Nov 1. Physiol Rep. 2014. PMID: 25367692 Free PMC article.
References
-
- Breunig JJ, Macklis JD, Rakic P. Evloving methods for the labeling and mutation of postnatal neuronal precursor cells: a critical review. Adult neurogenesis. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 49–80.
-
- Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A. Detection of S–phase cell cycle progression using 5–ethynyl–2′–deoxyuridine incorporation with click chemistry, an alternative to using 5–bromo–2′–deoxyuridine antibodies. Biotechniques. 2008;44:927–929. - PubMed
-
- Burns KA, Kuan CY. Low doses of bromo– and iododeoxyuridine produce near–saturation labeling of adult proliferative populations in the dentate gyrus. Eur J Neurosci. 2005;21:803–807. - PubMed
-
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. - PubMed
-
- Cappella P, Gasparri F, Pulici M, Moll J. A novel method based on click chemistry, which overcomes limitations of cell cycle analysis by classical determination of BrdU incorporation, allowing multiplex antibody staining. Cytometry A. 2008;73:626–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

