Intact fetal ovarian cord formation promotes mouse oocyte survival and development
- PMID: 20064216
- PMCID: PMC2830955
- DOI: 10.1186/1471-213X-10-2
Intact fetal ovarian cord formation promotes mouse oocyte survival and development
Abstract
Background: Female reproductive potential, or the ability to propagate life, is limited in mammals with the majority of oocytes lost before birth. In mice, surviving perinatal oocytes are enclosed in ovarian follicles for subsequent oocyte development and function in the adult. Before birth, fetal germ cells of both sexes develop in clusters, or germline cysts, in the undifferentiated gonad. Upon sex determination of the fetal gonad, germ cell cysts become organized into testicular or ovarian cord-like structures and begin to interact with gonadal somatic cells. Although germline cysts and testicular cords are required for spermatogenesis, the role of cyst and ovarian cord formation in mammalian oocyte development and female fertility has not been determined.
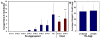
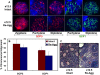
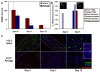
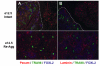
Results: Here, we examine whether intact fetal ovarian germ and somatic cell cord structures are required for oocyte development using mouse gonad re-aggregation and transplantation to disrupt gonadal organization. We observed that germ cells from disrupted female gonad prior to embryonic day e13.5 completed prophase I of meiosis but did not survive following transplantation. Furthermore, re-aggregated ovaries from e13.5 to e15.5 developed with a reduced number of oocytes. Oocyte loss occurred before follicle formation and was associated with an absence of ovarian cord structure and ovary disorganization. However, disrupted ovaries from e16.5 or later were resistant to the re-aggregation impairment and supported robust oocyte survival and development in follicles.
Conclusions: Thus, we demonstrate a critical window of oocyte development from e13.5 to e16.5 in the intact fetal mouse ovary, corresponding to the establishment of ovarian cord structure, which promotes oocyte interaction with neighboring ovarian somatic granulosa cells before birth and imparts oocytes with competence to survive and develop in follicles. Because germline cyst and ovarian cord structures are conserved in the human fetal ovary, the identification of genetic components and molecular mechanisms of pre-follicle stage germ and somatic cell structures may be important for understanding human female infertility. In addition, this work provides a foundation for development of a robust fetal ovarian niche and transplantation based system to direct stem cell-derived oocyte differentiation as a potential therapeutic strategy for the treatment of infertility.
Figures




Similar articles
-
Beyond apoptosis: evidence of other regulated cell death pathways in the ovary throughout development and life.Hum Reprod Update. 2023 Jul 5;29(4):434-456. doi: 10.1093/humupd/dmad005. Hum Reprod Update. 2023. PMID: 36857094 Free PMC article. Review.
-
Molecular analysis of the effects of steroid hormones on mouse meiotic prophase I progression.Reprod Biol Endocrinol. 2019 Dec 2;17(1):105. doi: 10.1186/s12958-019-0548-x. Reprod Biol Endocrinol. 2019. PMID: 31791345 Free PMC article.
-
Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles.BMC Biol. 2013 Feb 13;11:13. doi: 10.1186/1741-7007-11-13. BMC Biol. 2013. PMID: 23406467 Free PMC article.
-
Interaction between oocytes, cortical germ cells and granulosa cells of the mouse and bat, following the dissociation-re-aggregation of adult ovaries.Zygote. 2020 Jun;28(3):223-232. doi: 10.1017/S0967199420000052. Epub 2020 Mar 3. Zygote. 2020. PMID: 32122435
-
[Reconsidering the roles of female germ cells in ovarian development and folliculogenesis].Biol Aujourdhui. 2011;205(4):223-33. doi: 10.1051/jbio/2011022. Epub 2012 Jan 19. Biol Aujourdhui. 2011. PMID: 22251857 Review. French.
Cited by
-
Ovarian regeneration: The potential for stem cell contribution in the postnatal ovary to sustained endocrine function.Mol Cell Endocrinol. 2017 Apr 15;445:74-84. doi: 10.1016/j.mce.2016.10.012. Epub 2016 Oct 12. Mol Cell Endocrinol. 2017. PMID: 27743990 Free PMC article. Review.
-
Ovarian adult stem cells: hope or pitfall?J Ovarian Res. 2014 Jul 4;7:71. doi: 10.1186/1757-2215-7-71. eCollection 2014. J Ovarian Res. 2014. PMID: 25018783 Free PMC article. Review.
-
Defining the neighborhoods that escort the oocyte through its early life events and into a functional follicle.Mol Reprod Dev. 2013 Dec;80(12):960-76. doi: 10.1002/mrd.22232. Epub 2013 Sep 16. Mol Reprod Dev. 2013. PMID: 24105719 Free PMC article. Review.
-
Mouse Ovarian Very Small Embryonic-Like Stem Cells Resist Chemotherapy and Retain Ability to Initiate Oocyte-Specific Differentiation.Reprod Sci. 2015 Jul;22(7):884-903. doi: 10.1177/1933719115576727. Epub 2015 Mar 16. Reprod Sci. 2015. PMID: 25779995 Free PMC article.
-
Ovulation: Parallels With Inflammatory Processes.Endocr Rev. 2019 Apr 1;40(2):369-416. doi: 10.1210/er.2018-00075. Endocr Rev. 2019. PMID: 30496379 Free PMC article. Review.
References
-
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev. 1986;66:71–117. - PubMed
-
- Gondos B, Zamboni L. Ovarian development: the functional importance of germ cell interconnections. Fertil Steril. 1969;20:176–89. - PubMed
-
- de Cuevas M, Lee JK, Spradling AC. alpha-spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development. 1996;122:3959–68. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

