Simian TRIM5alpha proteins reduce replication of herpes simplex virus
- PMID: 20060996
- PMCID: PMC2823815
- DOI: 10.1016/j.virol.2009.11.041
Simian TRIM5alpha proteins reduce replication of herpes simplex virus
Abstract
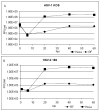
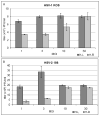
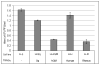
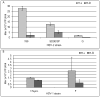
Old World monkey TRIM5alpha proteins are known to block the replication of human immunodeficiency virus and other retroviruses in a species-specific fashion. In this report, we show that specific forms of simian TRIM5alpha proteins can restrict herpes simplex virus (HSV) infection. To define the effect of TRIM5alpha on HSV replication, we examined HSV infection in HeLa cell lines that stably express simian and human orthologs of TRIM5alpha proteins. We demonstrated that several simian TRIM5alpha proteins can restrict HSV replication, with the TRIM5alpha protein of rhesus macaques showing the strongest inhibition of HSV infection. We also found that the level of the inhibition of virus replication was viral strain-specific. TRIM5alpha is likely to inhibit HSV at the early stage of infection; however, at later times of infection, the levels of TRIM5alpha are significantly decreased. Thus, some TRIM5alpha proteins exhibit antiviral effects that extend beyond retroviral infections, but HSV may be able to reduce this restriction by reducing TRIM5alpha levels during the later phases of virus replication. Our results also argue that TRIM5alpha is only part of the reduced level of HSV replication in rhesus macaques, which are known to be less susceptible to HSV infection than other primates.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
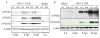



Similar articles
-
H2AX phosphorylation and DNA damage kinase activity are dispensable for herpes simplex virus replication.Virol J. 2016 Jan 27;13:15. doi: 10.1186/s12985-016-0470-1. Virol J. 2016. PMID: 26817608 Free PMC article.
-
The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys.Nature. 2004 Feb 26;427(6977):848-53. doi: 10.1038/nature02343. Nature. 2004. PMID: 14985764
-
Novel Role for Protein Inhibitor of Activated STAT 4 (PIAS4) in the Restriction of Herpes Simplex Virus 1 by the Cellular Intrinsic Antiviral Immune Response.J Virol. 2016 Apr 14;90(9):4807-4826. doi: 10.1128/JVI.03055-15. Print 2016 May. J Virol. 2016. PMID: 26937035 Free PMC article.
-
Herpes simplex virus.Front Biosci. 2002 Mar 1;7:d752-64. doi: 10.2741/taylor. Front Biosci. 2002. PMID: 11861220 Review.
-
Viral, neuronal and immune factors which may influence herpes simplex virus (HSV) latency and reactivation.Microb Pathog. 1993 Aug;15(2):83-91. doi: 10.1006/mpat.1993.1059. Microb Pathog. 1993. PMID: 8255209 Review. No abstract available.
Cited by
-
Equine Alphaherpesviruses Require Activation of the Small GTPases Rac1 and Cdc42 for Intracellular Transport.Microorganisms. 2020 Jul 7;8(7):1013. doi: 10.3390/microorganisms8071013. Microorganisms. 2020. PMID: 32645930 Free PMC article.
-
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1.Microbiol Mol Biol Rev. 2020 Sep 30;84(4):e00099-20. doi: 10.1128/MMBR.00099-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 32998978 Free PMC article. Review.
-
Comparing chemical transfection, electroporation, and lentiviral vector transduction to achieve optimal transfection conditions in the Vero cell line.BMC Mol Cell Biol. 2024 May 13;25(1):15. doi: 10.1186/s12860-024-00511-x. BMC Mol Cell Biol. 2024. PMID: 38741034 Free PMC article.
-
TRIM5α Promotes Ubiquitination of Rta from Epstein-Barr Virus to Attenuate Lytic Progression.Front Microbiol. 2017 Jan 5;7:2129. doi: 10.3389/fmicb.2016.02129. eCollection 2016. Front Microbiol. 2017. PMID: 28105027 Free PMC article.
-
Hijacking of the Ubiquitin/Proteasome Pathway by the HIV Auxiliary Proteins.Viruses. 2017 Oct 31;9(11):322. doi: 10.3390/v9110322. Viruses. 2017. PMID: 29088112 Free PMC article. Review.
References
-
- Blondel D, Regad T, Poisson N, Pavie B, Harper F, Pandolfi PP, De Thé H, Chelbi-Alix MK. Rabies virus P and small P products interact directly with PML and reorganize PML nuclear bodies. Oncogene. 2002;21:7957–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

