Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein
- PMID: 20053882
- PMCID: PMC6632534
- DOI: 10.1523/JNEUROSCI.4074-09.2010
Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein
Abstract
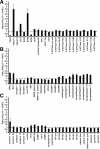
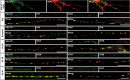
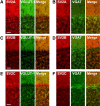
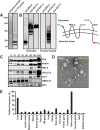
Synaptic vesicles (SVs) store neurotransmitters and release them by exocytosis. The vesicular neurotransmitter transporters discriminate which transmitter will be sequestered and stored by the vesicles. However, it is unclear whether the neurotransmitter phenotype of SVs is solely defined by the transporters or whether it is associated with additional proteins. Here we have compared the protein composition of SVs enriched in vesicular glutamate (VGLUT-1) and GABA transporters (VGAT), respectively, using quantitative proteomics. Of >450 quantified proteins, approximately 50 were differentially distributed between the populations, with only few of them being specific for SVs. Of these, the most striking differences were observed for the zinc transporter ZnT3 and the vesicle proteins SV2B and SV31 that are associated preferentially with VGLUT-1 vesicles, and for SV2C that is associated mainly with VGAT vesicles. Several additional proteins displayed a preference for VGLUT-1 vesicles including, surprisingly, synaptophysin, synaptotagmins, and syntaxin 1a. Moreover, MAL2, a membrane protein of unknown function distantly related to synaptophysins and SCAMPs, cofractionated with VGLUT-1 vesicles. Both subcellular fractionation and immunolocalization at the light and electron microscopic level revealed that MAL2 is a bona-fide membrane constituent of SVs that is preferentially associated with VGLUT-1-containing nerve terminals. We conclude that SVs specific for different neurotransmitters share the majority of their protein constituents, with only few vesicle proteins showing preferences that, however, are nonexclusive, thus confirming that the vesicular transporters are the only components essential for defining the neurotransmitter phenotype of a SV.
Figures



Similar articles
-
Unique pH dynamics in GABAergic synaptic vesicles illuminates the mechanism and kinetics of GABA loading.Proc Natl Acad Sci U S A. 2016 Sep 20;113(38):10702-7. doi: 10.1073/pnas.1604527113. Epub 2016 Sep 6. Proc Natl Acad Sci U S A. 2016. PMID: 27601664 Free PMC article.
-
Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex: analysis of synaptogyrin, vesicle-associated membrane protein, and syntaxin.Neuroscience. 2010 Feb 3;165(3):934-43. doi: 10.1016/j.neuroscience.2009.11.009. Epub 2009 Nov 10. Neuroscience. 2010. PMID: 19909789
-
The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons.J Neurosci. 1998 Dec 1;18(23):9733-50. doi: 10.1523/JNEUROSCI.18-23-09733.1998. J Neurosci. 1998. PMID: 9822734 Free PMC article.
-
Synaptic vesicle protein trafficking at the glutamate synapse.Neuroscience. 2009 Jan 12;158(1):189-203. doi: 10.1016/j.neuroscience.2008.03.029. Epub 2008 Mar 22. Neuroscience. 2009. PMID: 18472224 Free PMC article. Review.
-
Vesicular neurotransmitter transport and the presynaptic regulation of quantal size.Curr Opin Neurobiol. 1998 Jun;8(3):405-12. doi: 10.1016/s0959-4388(98)80068-8. Curr Opin Neurobiol. 1998. PMID: 9687352 Review.
Cited by
-
Presynaptic regulation of quantal size: K+/H+ exchange stimulates vesicular glutamate transport.Nat Neurosci. 2011 Aug 28;14(10):1285-92. doi: 10.1038/nn.2898. Nat Neurosci. 2011. PMID: 21874016 Free PMC article.
-
Tuning of Glutamate, But Not GABA, Release by an Intrasynaptic Vesicle APP Domain Whose Function Can Be Modulated by β- or α-Secretase Cleavage.J Neurosci. 2019 Aug 28;39(35):6992-7005. doi: 10.1523/JNEUROSCI.0207-19.2019. Epub 2019 Jun 24. J Neurosci. 2019. PMID: 31235642 Free PMC article.
-
Structure and topography of the synaptic V-ATPase-synaptophysin complex.Nature. 2024 Jul;631(8022):899-904. doi: 10.1038/s41586-024-07610-x. Epub 2024 Jun 5. Nature. 2024. PMID: 38838737 Free PMC article.
-
Functionally heterogeneous synaptic vesicle pools support diverse synaptic signalling.J Physiol. 2016 Feb 15;594(4):825-35. doi: 10.1113/JP270194. Epub 2015 Dec 28. J Physiol. 2016. PMID: 26614712 Free PMC article. Review.
-
Presynaptic Molecular Determinants of Quantal Size.Front Synaptic Neurosci. 2016 Feb 8;8:2. doi: 10.3389/fnsyn.2016.00002. eCollection 2016. Front Synaptic Neurosci. 2016. PMID: 26903855 Free PMC article. Review.
References
-
- Aguado F, Majó G, Ruiz-Montasell B, Llorens J, Marsal J, Blasi J. Syntaxin 1A and 1B display distinct distribution patterns in the rat peripheral nervous system. Neuroscience. 1999;88:437–446. - PubMed
-
- Ahnert-Hilger G, Höltje M, Pahner I, Winter S, Brunk I. Regulation of vesicular neurotransmitter transporters. Rev Physiol Biochem Pharmacol. 2003;150:140–160. - PubMed
-
- Bähler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
