Chk1 haploinsufficiency results in anemia and defective erythropoiesis
- PMID: 20052416
- PMCID: PMC2798715
- DOI: 10.1371/journal.pone.0008581
Chk1 haploinsufficiency results in anemia and defective erythropoiesis
Abstract
Background: Erythropoiesis is a highly regulated and well-characterized developmental process responsible for providing the oxygen transport system of the body. However, few of the mechanisms involved in this process have been elucidated. Checkpoint Kinase 1 (Chk1) is best known for its role in the cell cycle and DNA damage pathways, and it has been shown to play a part in several pathways which when disrupted can lead to anemia.
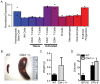
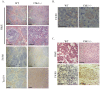
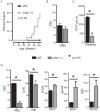
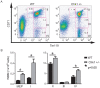
Methodology/principal findings: Here, we show that haploinsufficiency of Chk1 results in 30% of mice developing anemia within the first year of life. The anemic Chk1+/- mice exhibit distorted spleen and bone marrow architecture, and abnormal erythroid progenitors. Furthermore, Chk1+/- erythroid progenitors exhibit an increase in spontaneous DNA damage foci and improper contractile actin ring formation resulting in aberrant enucleation during erythropoiesis. A decrease in Chk1 RNA has also been observed in patients with refractory anemia with excess blasts, further supporting a role for Chk1 in clinical anemia.
Conclusions/significance: Clinical trials of Chk1 inhibitors are currently underway to treat cancer, and thus it will be important to track the effects of these drugs on red blood cell development over an extended period. Our results support a role for Chk1 in maintaining the balance between erythroid progenitors and enucleated erythroid cells during differentiation. We show disruptions in Chk1 levels can lead to anemia.
Conflict of interest statement
Figures
Similar articles
-
Mutant KLF1 in Adult Anemic Nan Mice Leads to Profound Transcriptome Changes and Disordered Erythropoiesis.Sci Rep. 2018 Aug 24;8(1):12793. doi: 10.1038/s41598-018-30839-2. Sci Rep. 2018. PMID: 30143664 Free PMC article.
-
Transient knock down of checkpoint kinase 1 in hematopoietic progenitors is linked to bone marrow toxicity.Toxicol Lett. 2011 Jul 28;204(2-3):141-7. doi: 10.1016/j.toxlet.2011.04.025. Epub 2011 Apr 30. Toxicol Lett. 2011. PMID: 21557990
-
A KIT juxtamembrane PY567 -directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis.Exp Hematol. 2009 Feb;37(2):159-71. doi: 10.1016/j.exphem.2008.10.009. Epub 2008 Dec 18. Exp Hematol. 2009. PMID: 19100679 Free PMC article.
-
Apoptotic mechanisms in the control of erythropoiesis.Leukemia. 2004 Jul;18(7):1176-99. doi: 10.1038/sj.leu.2403383. Leukemia. 2004. PMID: 15208642 Review.
-
The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability.Front Biosci. 2008 May 1;13:5016-29. doi: 10.2741/3060. Front Biosci. 2008. PMID: 18508566 Review.
Cited by
-
Checkpoint kinase 1 is essential for fetal and adult hematopoiesis.EMBO Rep. 2019 Aug;20(8):e47026. doi: 10.15252/embr.201847026. Epub 2019 Jun 17. EMBO Rep. 2019. PMID: 31379128 Free PMC article.
-
Phase I dose-escalation study to examine the safety and tolerability of LY2603618, a checkpoint 1 kinase inhibitor, administered 1 day after pemetrexed 500 mg/m(2) every 21 days in patients with cancer.Invest New Drugs. 2013 Feb;31(1):136-44. doi: 10.1007/s10637-012-9815-9. Epub 2012 Apr 11. Invest New Drugs. 2013. PMID: 22492020 Free PMC article. Clinical Trial.
-
Evaluation of checkpoint kinase targeting therapy in acute myeloid leukemia with complex karyotype.Cancer Biol Ther. 2012 Mar;13(5):307-13. doi: 10.4161/cbt.19074. Epub 2012 Mar 1. Cancer Biol Ther. 2012. PMID: 22258035 Free PMC article.
-
Small molecule inhibitors and a kinase-dead expressing mouse model demonstrate that the kinase activity of Chk1 is essential for mouse embryos and cancer cells.Life Sci Alliance. 2020 Jun 22;3(8):e202000671. doi: 10.26508/lsa.202000671. Print 2020 Aug. Life Sci Alliance. 2020. PMID: 32571801 Free PMC article.
-
Ataxin-3 promotes genome integrity by stabilizing Chk1.Nucleic Acids Res. 2017 May 5;45(8):4532-4549. doi: 10.1093/nar/gkx095. Nucleic Acids Res. 2017. PMID: 28180282 Free PMC article.
References
-
- Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9:93–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

