Molecular aspects of thyroid hormone actions
- PMID: 20051527
- PMCID: PMC2852208
- DOI: 10.1210/er.2009-0007
Molecular aspects of thyroid hormone actions
Abstract
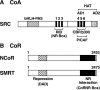
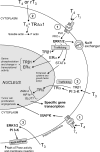
Cellular actions of thyroid hormone may be initiated within the cell nucleus, at the plasma membrane, in cytoplasm, and at the mitochondrion. Thyroid hormone nuclear receptors (TRs) mediate the biological activities of T(3) via transcriptional regulation. Two TR genes, alpha and beta, encode four T(3)-binding receptor isoforms (alpha1, beta1, beta2, and beta3). The transcriptional activity of TRs is regulated at multiple levels. Besides being regulated by T(3), transcriptional activity is regulated by the type of thyroid hormone response elements located on the promoters of T(3) target genes, by the developmental- and tissue-dependent expression of TR isoforms, and by a host of nuclear coregulatory proteins. These nuclear coregulatory proteins modulate the transcription activity of TRs in a T(3)-dependent manner. In the absence of T(3), corepressors act to repress the basal transcriptional activity, whereas in the presence of T(3), coactivators function to activate transcription. The critical role of TRs is evident in that mutations of the TRbeta gene cause resistance to thyroid hormones to exhibit an array of symptoms due to decreasing the sensitivity of target tissues to T(3). Genetically engineered knockin mouse models also reveal that mutations of the TRs could lead to other abnormalities beyond resistance to thyroid hormones, including thyroid cancer, pituitary tumors, dwarfism, and metabolic abnormalities. Thus, the deleterious effects of mutations of TRs are more severe than previously envisioned. These genetic-engineered mouse models provide valuable tools to ascertain further the molecular actions of unliganded TRs in vivo that could underlie the pathogenesis of hypothyroidism. Actions of thyroid hormone that are not initiated by liganding of the hormone to intranuclear TR are termed nongenomic. They may begin at the plasma membrane or in cytoplasm. Plasma membrane-initiated actions begin at a receptor on integrin alphavbeta3 that activates ERK1/2 and culminate in local membrane actions on ion transport systems, such as the Na(+)/H(+) exchanger, or complex cellular events such as cell proliferation. Concentration of the integrin on cells of the vasculature and on tumor cells explains recently described proangiogenic effects of iodothyronines and proliferative actions of thyroid hormone on certain cancer cells, including gliomas. Thus, hormonal events that begin nongenomically result in effects in DNA-dependent effects. l-T(4) is an agonist at the plasma membrane without conversion to T(3). Tetraiodothyroacetic acid is a T(4) analog that inhibits the actions of T(4) and T(3) at the integrin, including angiogenesis and tumor cell proliferation. T(3) can activate phosphatidylinositol 3-kinase by a mechanism that may be cytoplasmic in origin or may begin at integrin alphavbeta3. Downstream consequences of phosphatidylinositol 3-kinase activation by T(3) include specific gene transcription and insertion of Na, K-ATPase in the plasma membrane and modulation of the activity of the ATPase. Thyroid hormone, chiefly T(3) and diiodothyronine, has important effects on mitochondrial energetics and on the cytoskeleton. Modulation by the hormone of the basal proton leak in mitochondria accounts for heat production caused by iodothyronines and a substantial component of cellular oxygen consumption. Thyroid hormone also acts on the mitochondrial genome via imported isoforms of nuclear TRs to affect several mitochondrial transcription factors. Regulation of actin polymerization by T(4) and rT(3), but not T(3), is critical to cell migration. This effect has been prominently demonstrated in neurons and glial cells and is important to brain development. The actin-related effects in neurons include fostering neurite outgrowth. A truncated TRalpha1 isoform that resides in the extranuclear compartment mediates the action of thyroid hormone on the cytoskeleton.
Figures

Similar articles
-
Mechanisms of nongenomic actions of thyroid hormone.Front Neuroendocrinol. 2008 May;29(2):211-8. doi: 10.1016/j.yfrne.2007.09.003. Epub 2007 Oct 5. Front Neuroendocrinol. 2008. PMID: 17983645 Review.
-
Molecular Basis of Nongenomic Actions of Thyroid Hormone.Vitam Horm. 2018;106:67-96. doi: 10.1016/bs.vh.2017.06.001. Epub 2017 Jul 8. Vitam Horm. 2018. PMID: 29407448 Review.
-
Nongenomic actions of thyroid hormone.Nat Rev Endocrinol. 2016 Feb;12(2):111-21. doi: 10.1038/nrendo.2015.205. Epub 2015 Dec 15. Nat Rev Endocrinol. 2016. PMID: 26668118 Review.
-
Translational implications of nongenomic actions of thyroid hormone initiated at its integrin receptor.Am J Physiol Endocrinol Metab. 2009 Dec;297(6):E1238-46. doi: 10.1152/ajpendo.00480.2009. Epub 2009 Sep 15. Am J Physiol Endocrinol Metab. 2009. PMID: 19755667
-
Overlapping nongenomic and genomic actions of thyroid hormone and steroids.Best Pract Res Clin Endocrinol Metab. 2015 Aug;29(4):581-93. doi: 10.1016/j.beem.2015.04.001. Epub 2015 Apr 22. Best Pract Res Clin Endocrinol Metab. 2015. PMID: 26303085 Free PMC article. Review.
Cited by
-
Causal linkage of Graves' disease with aging: Mendelian randomization analysis of telomere length and age-related phenotypes.BMC Geriatr. 2024 Oct 31;24(1):901. doi: 10.1186/s12877-024-05379-2. BMC Geriatr. 2024. PMID: 39482583 Free PMC article.
-
Evidence-Based Use of Levothyroxine/Liothyronine Combinations in Treating Hypothyroidism: A Consensus Document.Eur Thyroid J. 2021 Mar;10(1):10-38. doi: 10.1159/000512970. Epub 2021 Feb 16. Eur Thyroid J. 2021. PMID: 33777817 Free PMC article.
-
A Role of Endogenous Histone Acetyltransferase Steroid Hormone Receptor Coactivator 3 in Thyroid Hormone Signaling During Xenopus Intestinal Metamorphosis.Thyroid. 2021 Apr;31(4):692-702. doi: 10.1089/thy.2020.0410. Epub 2020 Nov 20. Thyroid. 2021. PMID: 33076783 Free PMC article.
-
Influence of metabolic state and body composition on the action of pharmacological treatment of migraine.J Headache Pain. 2024 Feb 13;25(1):20. doi: 10.1186/s10194-024-01724-3. J Headache Pain. 2024. PMID: 38347465 Free PMC article. Review.
-
The thyroid hormone-αvβ3 integrin axis in ovarian cancer: regulation of gene transcription and MAPK-dependent proliferation.Oncogene. 2016 Apr 14;35(15):1977-87. doi: 10.1038/onc.2015.262. Epub 2015 Jul 13. Oncogene. 2016. PMID: 26165836
References
-
- Oppenheimer JH, Schwartz HL, Surks MI 1974 Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology 95:897–903 - PubMed
-
- Samuels HH, Tsai JS, Casanova J 1974 Thyroid hormone action: in vitro demonstration of putative receptors in isolated nuclei and soluble nuclear extracts. Science 184:1188–1191 - PubMed
-
- Latham KR, Ring JC, Baxter JD 1976 Solubilized nuclear “receptors” for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem 251:7388–7397 - PubMed
-
- Apriletti JW, Eberhardt NL, Latham KR, Baxter JD 1981 Affinity chromatography of thyroid hormone receptors. Biospecific elution from support matrices, characterization of the partially purified receptor. J Biol Chem 256:12094–12101 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

