Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes
- PMID: 20051513
- PMCID: PMC2844211
- DOI: 10.1074/jbc.M109.058990
Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes
Abstract
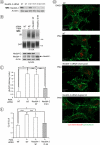
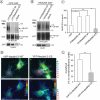
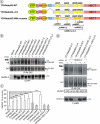
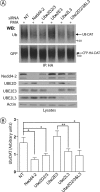
RNA interference screen previously revealed that a HECT-domain E3 ubiquitin ligase, neuronal precursor cell expressed, developmentally down-regulated 4-2 (Nedd4-2), is necessary for ubiquitination and endocytosis of the dopamine transporter (DAT) induced by the activation of protein kinase C (PKC). To further confirm the role of Nedd4-2 in DAT ubiquitination and endocytosis, we demonstrated that the depletion of Nedd4-2 by two different small interfering RNA (siRNA) duplexes suppressed PKC-dependent ubiquitination and endocytosis of DAT in human and porcine cells, whereas knock-down of a highly homologous E3 ligase, Nedd4-1, had no effect on DAT. The abolished DAT ubiquitination in Nedd4-2-depleted cells was rescued by expression of recombinant Nedd4-2. Moreover, overexpression of Nedd4-2 resulted in increased PKC-dependent ubiquitination of DAT. Mutational inactivation of the HECT domain of Nedd4-2 inhibited DAT ubiquitination and endocytosis. Structure-function analysis of Nedd4-2-mediated DAT ubiquitination revealed that the intact WW4 domain and to a lesser extent WW3 domain are necessary for PKC-dependent DAT ubiquitination. Moreover, a fragment of the Nedd4-2 molecule containing WW3, WW4, and HECT domains was sufficient for fully potentiating PKC-dependent ubiquitination of DAT. Analysis of DAT ubiquitination using polyubiquitin chain-specific antibodies showed that DAT is mainly conjugated with Lys(63)-linked ubiquitin chains. siRNA analysis demonstrated that this polyubiquitination is mediated by Nedd4-2 cooperation with UBE2D and UBE2L3 E2 ubiquitin-conjugating enzymes. The model is proposed whereby each ubiquitinated DAT molecule is modified by a single four-ubiquitin Lys(63)-linked chain that can be conjugated to various lysine residues of DAT.
Figures

Similar articles
-
PKC/Nedd4-2 Signaling Pathway Regulates the Cell Surface Expression of Drug Transporter hOAT1.Drug Metab Dispos. 2017 Aug;45(8):887-895. doi: 10.1124/dmd.117.075861. Epub 2017 Jun 1. Drug Metab Dispos. 2017. PMID: 28572241 Free PMC article.
-
The role of Nedd4-1 WW domains in binding and regulating human organic anion transporter 1.Am J Physiol Renal Physiol. 2016 Aug 1;311(2):F320-9. doi: 10.1152/ajprenal.00153.2016. Epub 2016 May 25. Am J Physiol Renal Physiol. 2016. PMID: 27226107 Free PMC article.
-
Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation.J Biol Chem. 2012 Jun 1;287(23):19177-87. doi: 10.1074/jbc.M112.355909. Epub 2012 Apr 13. J Biol Chem. 2012. PMID: 22505712 Free PMC article.
-
Physiological Functions of the Ubiquitin Ligases Nedd4-1 and Nedd4-2.Physiology (Bethesda). 2024 Jan 1;39(1):18-29. doi: 10.1152/physiol.00023.2023. Epub 2023 Nov 14. Physiology (Bethesda). 2024. PMID: 37962894 Review.
-
NEDD4 E3 Ligases: Functions and Mechanisms in Bone and Tooth.Int J Mol Sci. 2022 Sep 1;23(17):9937. doi: 10.3390/ijms23179937. Int J Mol Sci. 2022. PMID: 36077334 Free PMC article. Review.
Cited by
-
Mechanisms of dopamine transporter regulation in normal and disease states.Trends Pharmacol Sci. 2013 Sep;34(9):489-96. doi: 10.1016/j.tips.2013.07.005. Epub 2013 Aug 20. Trends Pharmacol Sci. 2013. PMID: 23968642 Free PMC article. Review.
-
Locomotor hyperactivity in 14-3-3ζ KO mice is associated with dopamine transporter dysfunction.Transl Psychiatry. 2013 Dec 3;3(12):e327. doi: 10.1038/tp.2013.99. Transl Psychiatry. 2013. PMID: 24301645 Free PMC article.
-
Endosomal transport via ubiquitination.Trends Cell Biol. 2011 Nov;21(11):647-55. doi: 10.1016/j.tcb.2011.08.007. Epub 2011 Sep 28. Trends Cell Biol. 2011. PMID: 21955996 Free PMC article. Review.
-
RNA sequencing-based identification of the regulatory mechanism of microRNAs, transcription factors, and corresponding target genes involved in vascular dementia.Front Neurosci. 2022 Sep 20;16:917489. doi: 10.3389/fnins.2022.917489. eCollection 2022. Front Neurosci. 2022. PMID: 36203804 Free PMC article.
-
The Role of NEDD4 E3 Ubiquitin-Protein Ligases in Parkinson's Disease.Genes (Basel). 2022 Mar 14;13(3):513. doi: 10.3390/genes13030513. Genes (Basel). 2022. PMID: 35328067 Free PMC article. Review.
References
-
- Hicke L., Dunn R. (2003) Annu. Rev. Cell Dev. Biol. 19, 141–172 - PubMed
-
- Staub O., Rotin D. (2006) Physiol. Rev. 86, 669–707 - PubMed
-
- Mukhopadhyay D., Riezman H. (2007) Science 315, 201–205 - PubMed
-
- Woelk T., Oldrini B., Maspero E., Confalonieri S., Cavallaro E., Di Fiore P. P., Polo S. (2006) Nat. Cell Biol. 8, 1246–1254 - PubMed
-
- Mosesson Y., Yarden Y. (2006) Isr. Med. Assoc. J. 8, 233–237 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

