Structural insights into interferon regulatory factor activation
- PMID: 20043992
- PMCID: PMC2846214
- DOI: 10.1016/j.cellsig.2009.12.005
Structural insights into interferon regulatory factor activation
Abstract
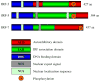
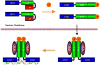
The interferon regulatory factors (IRFs) play important roles in development of the immune system and host defense. Recent crystallographic and biochemical studies have provided insights into the mechanism of activation of IRFs by phosphorylation. The activation of a latent closed conformation of IRF in the cytoplasm is triggered by phosphorylation of Ser/Thr residues in a C-terminal region. Phosphorylation stimulates the C-terminal autoinhibitory domain to attain a highly extended conformation triggering dimerization through extensive contacts to a second subunit. Dimers are then transported into the nucleus and assemble with the coactivator CBP/p300 to activate transcription of type I interferons and other target genes. The advances made in understanding the release of inhibition after IRF dimerization have generated a detailed structural model of how IRFs signaling pathways are activated.
Published by Elsevier Inc.
Figures

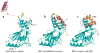
Similar articles
-
Insights into interferon regulatory factor activation from the crystal structure of dimeric IRF5.Nat Struct Mol Biol. 2008 Nov;15(11):1213-20. doi: 10.1038/nsmb.1496. Epub 2008 Oct 5. Nat Struct Mol Biol. 2008. PMID: 18836453 Free PMC article.
-
Rotavirus NSP1 mediates degradation of interferon regulatory factors through targeting of the dimerization domain.J Virol. 2013 Sep;87(17):9813-21. doi: 10.1128/JVI.01146-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824805 Free PMC article.
-
Direct Inhibition of IRF-Dependent Transcriptional Regulatory Mechanisms Associated With Disease.Front Immunol. 2019 May 24;10:1176. doi: 10.3389/fimmu.2019.01176. eCollection 2019. Front Immunol. 2019. PMID: 31178872 Free PMC article. Review.
-
Molecular characterization and expression analysis of eleven interferon regulatory factors in half-smooth tongue sole, Cynoglossus semilaevis.Fish Shellfish Immunol. 2015 May;44(1):272-82. doi: 10.1016/j.fsi.2015.02.033. Epub 2015 Feb 28. Fish Shellfish Immunol. 2015. PMID: 25731919
-
Targeting Interferon Regulatory Factor for Cardiometabolic Diseases: Opportunities and Challenges.Curr Drug Targets. 2017;18(15):1754-1778. doi: 10.2174/1389450116666150804110412. Curr Drug Targets. 2017. PMID: 26240052 Review.
Cited by
-
Pestivirus Npro Directly Interacts with Interferon Regulatory Factor 3 Monomer and Dimer.J Virol. 2016 Aug 12;90(17):7740-7. doi: 10.1128/JVI.00318-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334592 Free PMC article.
-
Genetic polymorphism of interferon regulatory factor 5 (IRF5) correlates with allograft acute rejection of liver transplantation.PLoS One. 2014 Apr 30;9(4):e94426. doi: 10.1371/journal.pone.0094426. eCollection 2014. PLoS One. 2014. Retraction in: PLoS One. 2019 Jul 31;14(7):e0220815. doi: 10.1371/journal.pone.0220815 PMID: 24788560 Free PMC article. Retracted.
-
Differential requirement of histone acetylase and deacetylase activities for IRF5-mediated proinflammatory cytokine expression.J Immunol. 2010 Nov 15;185(10):6003-12. doi: 10.4049/jimmunol.1000482. Epub 2010 Oct 8. J Immunol. 2010. PMID: 20935208 Free PMC article.
-
Avian IRF1 and IRF7 Play Overlapping and Distinct Roles in Regulating IFN-Dependent and -Independent Antiviral Responses to Duck Tembusu Virus Infection.Viruses. 2022 Jul 9;14(7):1506. doi: 10.3390/v14071506. Viruses. 2022. PMID: 35891486 Free PMC article.
-
Optineurin regulates the interferon response in a cell cycle-dependent manner.PLoS Pathog. 2015 Apr 29;11(4):e1004877. doi: 10.1371/journal.ppat.1004877. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25923723 Free PMC article.
References
-
- Tamura T, Yanai H, Savitsky D, Taniguchi T. Annu Rev Immunol. 2008;26:535–584. - PubMed
-
- Hu G, Mancl ME, Barnes BJ. Cancer Res. 2005;65(16):7403–7412. - PubMed
-
- Alter-Koltunoff M, Goren S, Nousbeck J, Feng CG, Sher A, Ozato K, Azriel A, Levi BZ. J Biol Chem. 2008;283(5):2724–2733. - PubMed
-
- Ozato K, Tailor P, Kubota T. J Biol Chem. 2007;282(28):20065–20069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

