The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events
- PMID: 20038533
- PMCID: PMC2820887
- DOI: 10.1128/MCB.01190-09
The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events
Abstract
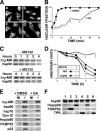
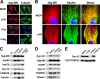
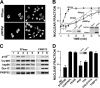
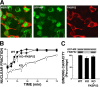
In this study, we demonstrate that the subcellular localization of the mineralocorticoid receptor (MR) is regulated by tetratricopeptide domain (TPR) proteins. The high-molecular-weight immunophilin (IMM) FKBP52 links the MR-hsp90 complex to dynein/dynactin motors favoring the cytoplasmic transport of MR to the nucleus. Replacement of this hsp90-binding IMM by FKBP51 or the TPR peptide favored the cytoplasmic localization of MR. The complete movement machinery, including dynein and tubulin, could be recovered from paclitaxel/GTP-stabilized cytosol and was fully reassembled on stripped MR immune pellets. The whole MR-hsp90-based heterocomplex was transiently recovered in the soluble fraction of the nucleus after 10 min of incubation with aldosterone. Moreover, cross-linked MR-hsp90 heterocomplexes accumulated in the nucleus in a hormone-dependent manner, demonstrating that the heterocomplex can pass undissociated through the nuclear pore. On the other hand, a peptide that comprises the DNA-binding domain of MR impaired the nuclear export of MR, suggesting the involvement of this domain in the process. This study represents the first report describing the entire molecular system that commands MR nucleocytoplasmic trafficking and proposes that the MR-hsp90-TPR protein heterocomplex is dissociated in the nucleus rather than in the cytoplasm.
Figures

Similar articles
-
Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity.Biochemistry. 2007 Dec 11;46(49):14044-57. doi: 10.1021/bi701372c. Epub 2007 Nov 15. Biochemistry. 2007. PMID: 18001136
-
Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain.Biochemistry. 2002 Nov 19;41(46):13602-10. doi: 10.1021/bi020399z. Biochemistry. 2002. PMID: 12427021
-
All of the protein interactions that link steroid receptor.hsp90.immunophilin heterocomplexes to cytoplasmic dynein are common to plant and animal cells.Biochemistry. 2002 Apr 30;41(17):5581-7. doi: 10.1021/bi020073q. Biochemistry. 2002. PMID: 11969419
-
Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52.Biomolecules. 2019 Feb 1;9(2):52. doi: 10.3390/biom9020052. Biomolecules. 2019. PMID: 30717249 Free PMC article. Review.
-
Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement.Cell Signal. 2004 Aug;16(8):857-72. doi: 10.1016/j.cellsig.2004.02.004. Cell Signal. 2004. PMID: 15157665 Review.
Cited by
-
Hsp90 co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged wild-type mice.Acta Neuropathol Commun. 2021 Apr 8;9(1):65. doi: 10.1186/s40478-021-01159-w. Acta Neuropathol Commun. 2021. PMID: 33832539 Free PMC article.
-
Importance of Micromilieu for Pathophysiologic Mineralocorticoid Receptor Activity-When the Mineralocorticoid Receptor Resides in the Wrong Neighborhood.Int J Mol Sci. 2022 Oct 20;23(20):12592. doi: 10.3390/ijms232012592. Int J Mol Sci. 2022. PMID: 36293446 Free PMC article. Review.
-
The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response.Arch Toxicol. 2021 Jun;95(6):1943-1970. doi: 10.1007/s00204-021-03070-8. Epub 2021 May 18. Arch Toxicol. 2021. PMID: 34003342 Review.
-
Management of cytoskeleton architecture by molecular chaperones and immunophilins.Cell Signal. 2011 Dec;23(12):1907-20. doi: 10.1016/j.cellsig.2011.07.023. Epub 2011 Aug 12. Cell Signal. 2011. PMID: 21864675 Free PMC article. Review.
-
Aldosterone: Renal Action and Physiological Effects.Compr Physiol. 2023 Mar 30;13(2):4409-4491. doi: 10.1002/cphy.c190043. Compr Physiol. 2023. PMID: 36994769 Free PMC article.
References
-
- Akner, G., K. Mossberg, K. G. Sundqvist, J. A. Gustafsson, and A. C. Wikstrom. 1992. Evidence for reversible, non-microtubule and non-microfilament-dependent nuclear translocation of hsp90 after heat shock in human fibroblasts. Eur. J. Cell Biol. 58:356-364. - PubMed
-
- Black, B. E., J. M. Holaska, F. Rastinejad, and B. M. Paschal. 2001. DNA binding domains in diverse nuclear receptors function as nuclear export signals. Curr. Biol. 11:1749-1758. - PubMed
-
- Buchholz, I., K. Enss, C. Schafer, A. Schlune, V. Shahin, and H. Oberleithner. 2004. Transient permeability leak of nuclear envelope induced by aldosterone. J. Membr. Biol. 199:135-141. - PubMed
-
- Cheung, P. Y., Y. Zhang, J. Long, S. Lin, M. Zhang, Y. Jiang, and Z. Wu. 2004. p150(Glued), dynein, and microtubules are specifically required for activation of MKK3/6 and p38 MAPKs. J. Biol. Chem. 279:45308-45311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
