Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes
- PMID: 20037153
- PMCID: PMC2820736
- DOI: 10.1074/jbc.C109.082727
Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes
Abstract
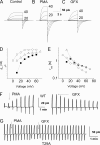
Voltage-gated proton channels and NADPH oxidase function cooperatively in phagocytes during the respiratory burst, when reactive oxygen species are produced to kill microbial invaders. Agents that activate NADPH oxidase also enhance proton channel gating profoundly, facilitating its roles in charge compensation and pH(i) regulation. The "enhanced gating mode" appears to reflect protein kinase C (PKC) phosphorylation. Here we examine two candidates for PKC-delta phosphorylation sites in the human voltage-gated proton channel, H(V)1 (Hvcn1), Thr(29) and Ser(97), both in the intracellular N terminus. Channel phosphorylation was reduced in single mutants S97A or T29A, and further in the double mutant T29A/S97A, by an in vitro kinase assay with PKC-delta. Enhanced gating was evaluated by expressing wild-type (WT) or mutant H(V)1 channels in LK35.2 cells, a B cell hybridoma. Stimulation by phorbol myristate acetate enhanced WT channel gating, and this effect was reversed by treatment with the PKC inhibitor GF109203X. The single mutant T29A or double mutant T29A/S97A failed to respond to phorbol myristate acetate or GF109203X. In contrast, the S97A mutant responded like cells transfected with WT H(V)1. We conclude that under these conditions, direct phosphorylation of the proton channel molecule at Thr(29) is primarily responsible for the enhancement of proton channel gating. This phosphorylation is crucial to activation of the proton conductance during the respiratory burst in phagocytes.
Figures
Similar articles
-
Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2 alpha activity.J Physiol. 2007 Mar 1;579(Pt 2):327-44. doi: 10.1113/jphysiol.2006.124248. Epub 2006 Dec 21. J Physiol. 2007. PMID: 17185330 Free PMC article.
-
Proton channel HVCN1 is required for effector functions of mouse eosinophils.BMC Immunol. 2013 May 24;14:24. doi: 10.1186/1471-2172-14-24. BMC Immunol. 2013. PMID: 23705768 Free PMC article.
-
The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps.J Biol Chem. 2001 Sep 28;276(39):36063-6. doi: 10.1074/jbc.C100352200. Epub 2001 Jul 26. J Biol Chem. 2001. PMID: 11477065
-
Pharmacology of voltage-gated proton channels.Curr Pharm Des. 2007;13(23):2400-20. doi: 10.2174/138161207781368675. Curr Pharm Des. 2007. PMID: 17692009 Review.
-
Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes.Physiology (Bethesda). 2010 Feb;25(1):27-40. doi: 10.1152/physiol.00039.2009. Physiology (Bethesda). 2010. PMID: 20134026 Free PMC article. Review.
Cited by
-
Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family.Physiol Rev. 2013 Apr;93(2):599-652. doi: 10.1152/physrev.00011.2012. Physiol Rev. 2013. PMID: 23589829 Free PMC article. Review.
-
A Review on the Role of Bicarbonate and Proton Transporters during Sperm Capacitation in Mammals.Int J Mol Sci. 2022 Jun 6;23(11):6333. doi: 10.3390/ijms23116333. Int J Mol Sci. 2022. PMID: 35683013 Free PMC article. Review.
-
Voltage-Gated Proton Channels in the Tree of Life.Biomolecules. 2023 Jun 24;13(7):1035. doi: 10.3390/biom13071035. Biomolecules. 2023. PMID: 37509071 Free PMC article. Review.
-
Rediscovering sperm ion channels with the patch-clamp technique.Mol Hum Reprod. 2011 Aug;17(8):478-99. doi: 10.1093/molehr/gar044. Epub 2011 Jun 4. Mol Hum Reprod. 2011. PMID: 21642646 Free PMC article. Review.
-
Role of voltage-gated proton channel (Hv1) in cancer biology.Front Pharmacol. 2023 Apr 20;14:1175702. doi: 10.3389/fphar.2023.1175702. eCollection 2023. Front Pharmacol. 2023. PMID: 37153807 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

