ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology
- PMID: 20035627
- PMCID: PMC2806266
- DOI: 10.1186/1742-2094-6-41
ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology
Abstract
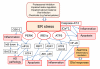
The endoplasmic reticulum (ER) is involved in several crucial cellular functions, e.g. protein folding and quality control, maintenance of Ca2+ balance, and cholesterol synthesis. Many genetic and environmental insults can disturb the function of ER and induce ER stress. ER contains three branches of stress sensors, i.e. IRE1, PERK and ATF6 transducers, which recognize the misfolding of proteins in ER and activate a complex signaling network to generate the unfolded protein response (UPR). Alzheimer's disease (AD) is a progressive neurodegenerative disorder involving misfolding and aggregation of proteins in conjunction with prolonged cellular stress, e.g. in redox regulation and Ca2+ homeostasis. Emerging evidence indicates that the UPR is activated in neurons but not in glial cells in AD brains. Neurons display pPERK, peIF2alpha and pIRE1alpha immunostaining along with abundant diffuse staining of phosphorylated tau protein. Recent studies have demonstrated that ER stress can also induce an inflammatory response via different UPR transducers. The most potent pathways are IRE1-TRAF2, PERK-eIF2alpha, PERK-GSK-3, ATF6-CREBH, as well as inflammatory caspase-induced signaling pathways. We will describe the mechanisms which could link the ER stress of neurons to the activation of the inflammatory response and the evolution of pathological changes in AD.
Figures
Similar articles
-
Endoplasmic reticulum stress in Alzheimer's disease: Molecular mechanisms and therapeutic prospects.Life Sci. 2023 Oct 1;330:121983. doi: 10.1016/j.lfs.2023.121983. Epub 2023 Jul 29. Life Sci. 2023. PMID: 37524162 Review.
-
Endoplasmic reticulum dysfunction in Alzheimer's disease.Mol Neurobiol. 2015 Feb;51(1):383-95. doi: 10.1007/s12035-014-8695-8. Epub 2014 Apr 9. Mol Neurobiol. 2015. PMID: 24715417 Review.
-
ER stress activates immunosuppressive network: implications for aging and Alzheimer's disease.J Mol Med (Berl). 2020 May;98(5):633-650. doi: 10.1007/s00109-020-01904-z. Epub 2020 Apr 11. J Mol Med (Berl). 2020. PMID: 32279085 Free PMC article. Review.
-
Induction of neuronal death by ER stress in Alzheimer's disease.J Chem Neuroanat. 2004 Sep;28(1-2):67-78. doi: 10.1016/j.jchemneu.2003.12.004. J Chem Neuroanat. 2004. PMID: 15363492 Review.
-
Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer's disease.FEBS J. 2018 Mar;285(6):995-1011. doi: 10.1111/febs.14332. Epub 2017 Dec 15. FEBS J. 2018. PMID: 29148236 Review.
Cited by
-
Dysfunctional Sensory Modalities, Locus Coeruleus, and Basal Forebrain: Early Determinants that Promote Neuropathogenesis of Cognitive and Memory Decline and Alzheimer's Disease.Neurotox Res. 2016 Oct;30(3):295-337. doi: 10.1007/s12640-016-9643-3. Epub 2016 Jun 23. Neurotox Res. 2016. PMID: 27339162 Review.
-
Non-coding RNAs in Alzheimer's disease.Mol Neurobiol. 2013 Feb;47(1):382-93. doi: 10.1007/s12035-012-8359-5. Epub 2012 Oct 7. Mol Neurobiol. 2013. PMID: 23054683 Review.
-
Sal003 alleviated intervertebral disc degeneration by inhibiting apoptosis and extracellular matrix degradation through suppressing endoplasmic reticulum stress pathway in rats.Front Pharmacol. 2023 Jan 20;14:1095307. doi: 10.3389/fphar.2023.1095307. eCollection 2023. Front Pharmacol. 2023. PMID: 36744257 Free PMC article.
-
CurQ+, a Next-Generation Formulation of Curcumin, Ameliorates Growth Plate Chondrocyte Stress and Increases Limb Growth in a Mouse Model of Pseudoachondroplasia.Int J Mol Sci. 2023 Feb 14;24(4):3845. doi: 10.3390/ijms24043845. Int J Mol Sci. 2023. PMID: 36835255 Free PMC article.
-
Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review.Nutrients. 2018 Aug 4;10(8):1021. doi: 10.3390/nu10081021. Nutrients. 2018. PMID: 30081573 Free PMC article. Review.
References
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous