Laminin alpha4-null mutant mice develop chronic kidney disease with persistent overexpression of platelet-derived growth factor
- PMID: 20035058
- PMCID: PMC2808089
- DOI: 10.2353/ajpath.2010.090570
Laminin alpha4-null mutant mice develop chronic kidney disease with persistent overexpression of platelet-derived growth factor
Abstract
Each extracellular matrix compartment in the kidney has a unique composition, with regional specificity in the expression of various laminin isoforms. Although null mutations in the majority of laminin chains lead to specific developmental abnormalities in the kidney, Lama4-/- mice have progressive glomerular and tubulointerstitial fibrosis. These mice have a significant increase in expression of platelet-derived growth factor (PDGF)-BB, PDGF-DD, and PDGF receptor beta in association with immature glomerular and peritubular capillaries. In addition, mesangial cell exposure to alpha4-containing laminins, but not other isoforms, results in down-regulation of PDGF receptor mRNA and protein, suggesting a direct effect of LN411/LN421 on vessel maturation. Given the known role of overexpression of PDGF-BB and PDGF-DD on glomerular and tubulointerstitial fibrosis, these data suggest that failure of laminin alpha4-mediated down-regulation of PDGF activity contributes to the progressive renal lesions in this animal model. Given the recent demonstration that individuals with laminin alpha4 mutations develop cardiomyopathy, these findings may be relevant to kidney disease in humans.
Figures

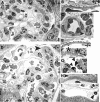
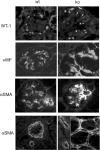
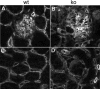
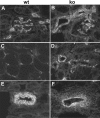

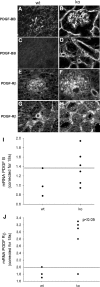
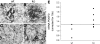
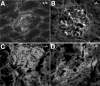
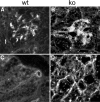

Similar articles
-
Laminin-8/9 is synthesized by rat glomerular mesangial cells and is required for PDGF-induced mesangial cell migration.Kidney Int. 2003 Jul;64(1):110-8. doi: 10.1046/j.1523-1755.2003.00039.x. Kidney Int. 2003. PMID: 12787401
-
Role of platelet-derived growth factor-CC in capillary rarefaction in renal fibrosis.Am J Pathol. 2015 Aug;185(8):2132-42. doi: 10.1016/j.ajpath.2015.04.022. Am J Pathol. 2015. PMID: 26216283
-
Complement C5 mediates experimental tubulointerstitial fibrosis.J Am Soc Nephrol. 2007 May;18(5):1508-15. doi: 10.1681/ASN.2006121343. Epub 2007 Mar 27. J Am Soc Nephrol. 2007. PMID: 17389734
-
The platelet-derived growth factor system in renal disease: an emerging role of endogenous inhibitors.Eur J Cell Biol. 2012 Jun-Jul;91(6-7):542-51. doi: 10.1016/j.ejcb.2011.07.003. Epub 2011 Aug 27. Eur J Cell Biol. 2012. PMID: 21872965 Review.
-
The PDGF family in renal fibrosis.Pediatr Nephrol. 2012 Jul;27(7):1041-50. doi: 10.1007/s00467-011-1892-z. Epub 2011 May 21. Pediatr Nephrol. 2012. PMID: 21597969 Review.
Cited by
-
The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis?Fibrogenesis Tissue Repair. 2014 Mar 28;7(1):4. doi: 10.1186/1755-1536-7-4. Fibrogenesis Tissue Repair. 2014. PMID: 24678881 Free PMC article.
-
Renal interstitial fibrosis: mechanisms and evaluation.Curr Opin Nephrol Hypertens. 2012 May;21(3):289-300. doi: 10.1097/MNH.0b013e3283521cfa. Curr Opin Nephrol Hypertens. 2012. PMID: 22449945 Free PMC article. Review.
-
Dual role of pericyte α6β1-integrin in tumour blood vessels.J Cell Sci. 2017 May 1;130(9):1583-1595. doi: 10.1242/jcs.197848. Epub 2017 Mar 13. J Cell Sci. 2017. PMID: 28289267 Free PMC article.
-
Adipocyte-Specific Laminin Alpha 4 Deletion Preserves Adipose Tissue Health despite Increasing Adiposity.Biomedicines. 2022 Aug 25;10(9):2077. doi: 10.3390/biomedicines10092077. Biomedicines. 2022. PMID: 36140178 Free PMC article.
-
Platelet-derived growth factors (PDGFs) in glomerular and tubulointerstitial fibrosis.Kidney Int Suppl (2011). 2014 Nov;4(1):65-69. doi: 10.1038/kisup.2014.12. Kidney Int Suppl (2011). 2014. PMID: 26312152 Free PMC article. Review.
References
-
- Kleinman HK, Weeks BS, Schnaper HW, Kibbey MC, Yamamura K, Grant DS. The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitamins Horm. 1993;47:161–186. - PubMed
-
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Tryggvason K, Yamada Y, Yurchenco PD. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. - PubMed
-
- Yamashita H, Beck K, Kitagawa Y. Heparin binds to the laminin α4 chain LG4 domain at a site different from that found for other laminins. J Mol Biol. 2004;335:1145–1149. - PubMed
-
- Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R. Structural and functional analysis of the recombinant G domain of the laminin α4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–35199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

