Circulating Th17, Th22, and Th1 cells are increased in psoriasis
- PMID: 20032993
- PMCID: PMC2892169
- DOI: 10.1038/jid.2009.399
Circulating Th17, Th22, and Th1 cells are increased in psoriasis
Abstract
Th17, Th22, and Th1 cells are detected in psoriatic skin lesions and implicated in psoriasis pathogenesis, but inflammatory T cell numbers in blood, as well as the relative importance of each cell type, is unclear. Using 7-color flow cytometry, circulating Th17, Th22, and Th1 cells were quantified in 21 untreated psoriatics and 17 healthy individuals. CCR6 was the best cell surface marker for IL-17A+ cells when compared with IL-23R or CD161. CCR6+, IL-17A+, IL-22+, CCR6+IL-17A+, CCR6+IL-22+, CCR6+tumor necrosis factor-alpha+, IL-17A+IFN-gamma-, IL-17A+IL-22+IFN-gamma-, and IL-17A+IL-22-IFN-gamma- cells were increased in psoriatics (all values P<0.001), indicating elevations in circulating Th17 cells, using multiple criteria to define these cells. Th22 (IL-17A-IL-22+IFN-gamma-, P<0.05) and Th1 (IL-17A-IFN-gamma+, P<0.05) cells were also increased in psoriatics, but to a lesser extent. Inhibition of either NF-kappaB or STAT3 in vitro blocked cytokine production by both Th17 and Th1 cells. Circulating levels of Th17 and Th1 cells decreased in a subset of five psoriasis patients serially evaluated following induction therapy with infliximab. In summary, elevated numbers of circulating inflammatory T cells may contribute to cutaneous inflammation and systemic inflammatory disease that occurs in individuals with psoriasis.
Figures
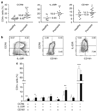


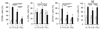
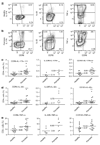
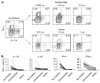
Similar articles
-
TH17/Treg lymphocyte balance is regulated by beta adrenergic and cAMP signaling.Brain Behav Immun. 2025 Jan;123:1061-1070. doi: 10.1016/j.bbi.2024.11.013. Epub 2024 Nov 13. Brain Behav Immun. 2025. PMID: 39542072
-
Comparison of On-Label Treatment Persistence in Real-World Patients with Psoriatic Arthritis Receiving Guselkumab Versus Subcutaneous Interleukin-17A Inhibitors.Adv Ther. 2024 Dec 2. doi: 10.1007/s12325-024-03042-1. Online ahead of print. Adv Ther. 2024. PMID: 39621228
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Interventions for nail psoriasis.Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD007633. doi: 10.1002/14651858.CD007633.pub2. Cochrane Database Syst Rev. 2013. PMID: 23440816 Free PMC article. Review.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
Cited by
-
IL-23/IL-17A Dysfunction Phenotypes Inform Possible Clinical Effects from Anti-IL-17A Therapies.J Invest Dermatol. 2015 Aug;135(8):1946-1953. doi: 10.1038/jid.2015.144. Epub 2015 Mar 24. J Invest Dermatol. 2015. PMID: 25972190 Free PMC article. Review.
-
Immune cells in the epithelial immune microenvironment of psoriasis: emerging therapeutic targets.Front Immunol. 2024 Jan 4;14:1340677. doi: 10.3389/fimmu.2023.1340677. eCollection 2023. Front Immunol. 2024. PMID: 38239345 Free PMC article. Review.
-
The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis.Clin Rev Allergy Immunol. 2018 Dec;55(3):379-390. doi: 10.1007/s12016-018-8702-3. Clin Rev Allergy Immunol. 2018. PMID: 30109481 Free PMC article. Review.
-
The Role of T Helper 22 Cells in Dermatological Disorders.Front Immunol. 2022 Jul 14;13:911546. doi: 10.3389/fimmu.2022.911546. eCollection 2022. Front Immunol. 2022. PMID: 35911703 Free PMC article. Review.
-
Small molecule allosteric inhibitors of RORγt block Th17-dependent inflammation and associated gene expression in vivo.PLoS One. 2021 Nov 9;16(11):e0248034. doi: 10.1371/journal.pone.0248034. eCollection 2021. PLoS One. 2021. PMID: 34752458 Free PMC article.
References
-
- Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. - PubMed
-
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

