Design and synthesis of selective inhibitors of placental alkaline phosphatase
- PMID: 20031422
- PMCID: PMC2818863
- DOI: 10.1016/j.bmc.2009.12.012
Design and synthesis of selective inhibitors of placental alkaline phosphatase
Abstract
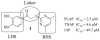
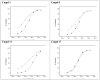
Placental Alkaline Phosphatase (PLAP) is a tissue-restricted isozyme of the Alkaline Phosphatase (AP) superfamily. PLAP is an oncodevelopmental enzyme expressed during pregnancy and in a variety of human cancers, but its biological function remains unknown. We report here a series of catechol compounds with great affinity for the PLAP isozyme and significant selectivity over other members of the AP superfamily. These selective PLAP inhibitors will provide small molecule probes for the study of the pathophysiological role of PLAP.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
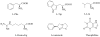
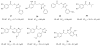
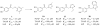

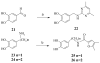
Similar articles
-
Modulators of intestinal alkaline phosphatase.Methods Mol Biol. 2013;1053:135-44. doi: 10.1007/978-1-62703-562-0_9. Methods Mol Biol. 2013. PMID: 23860652
-
Specific Inhibitor of Placental Alkaline Phosphatase Isolated from a DNA-Encoded Chemical Library Targets Tumor of the Female Reproductive Tract.J Med Chem. 2021 Nov 11;64(21):15799-15809. doi: 10.1021/acs.jmedchem.1c01103. Epub 2021 Oct 28. J Med Chem. 2021. PMID: 34709820
-
Recent advances with alkaline phosphatase isoenzymes and their inhibitors.Arch Pharm (Weinheim). 2020 May;353(5):e2000011. doi: 10.1002/ardp.202000011. Epub 2020 Mar 4. Arch Pharm (Weinheim). 2020. PMID: 32128876 Review.
-
Hybrid compounds from chalcone and 1,2-benzothiazine pharmacophores as selective inhibitors of alkaline phosphatase isozymes.Eur J Med Chem. 2018 Nov 5;159:282-291. doi: 10.1016/j.ejmech.2018.09.063. Epub 2018 Sep 26. Eur J Med Chem. 2018. PMID: 30296687
-
Placental alkaline phosphatase as the placental IgG receptor.Clin Chem. 1992 Dec;38(12):2543-5. Clin Chem. 1992. PMID: 1458596 Review.
Cited by
-
2-Benzylidenebenzofuran-3(2H)-ones as a new class of alkaline phosphatase inhibitors: synthesis, SAR analysis, enzyme inhibitory kinetics and computational studies.RSC Adv. 2021 Oct 29;11(56):35077-35092. doi: 10.1039/d1ra07379f. eCollection 2021 Oct 28. RSC Adv. 2021. PMID: 35493176 Free PMC article.
-
Assay format as a critical success factor for identification of novel inhibitor chemotypes of tissue-nonspecific alkaline phosphatase from high-throughput screening.Molecules. 2010 Apr 27;15(5):3010-37. doi: 10.3390/molecules15053010. Molecules. 2010. PMID: 20657462 Free PMC article. Review.
-
Ectonucleotidase inhibitors: targeting signaling pathways for therapeutic advancement-an in-depth review.Purinergic Signal. 2024 Jul 3. doi: 10.1007/s11302-024-10031-0. Online ahead of print. Purinergic Signal. 2024. PMID: 38958821 Review.
-
Current status of N-, O-, S-heterocycles as potential alkaline phosphatase inhibitors: a medicinal chemistry overview.RSC Adv. 2023 Jun 1;13(24):16413-16452. doi: 10.1039/d3ra01888a. eCollection 2023 May 30. RSC Adv. 2023. PMID: 37274413 Free PMC article. Review.
-
Proteoliposomes as matrix vesicles' biomimetics to study the initiation of skeletal mineralization.Braz J Med Biol Res. 2010 Mar;43(3):234-41. doi: 10.1590/s0100-879x2010007500008. Braz J Med Biol Res. 2010. PMID: 20401430 Free PMC article. Review.
References
-
- Millán JL. Mammalian alkaline phosphatases. From Biology to Applications in Medicine and Biotechnology. Wiley-VCH Verlag GmbH & Co; Weinheim, Germany: 2006. pp. 1–322.
-
- McComb RB, Bowers GN, Jr, Posen S. Alkaline Phosphatase. New York: Plenum; 1979.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

