A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland
- PMID: 20029965
- PMCID: PMC2819579
- DOI: 10.1002/dvg.20586
A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland
Abstract
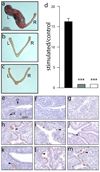
Considering the regulatory complexities of progesterone receptor (PR) action throughout the female reproductive axis and mammary gland, we generated a mouse model that enables conditional ablation of PR function in a spatiotemporal specific manner. Exon 2 of the murine PR gene was floxed to generate a conditional PR allele (PR(flox)) in mice. Crossing the PR(flox/flox) mouse with the ZP3-cre transgenic demonstrated that the PR(flox) allele recombines to a PR null allele (PR(d)). Mice homozygous for the recombined null PR allele (PR(d/d)) exhibit uterine, ovarian, and mammary gland defects that phenocopy those of our previously described PR knockout (PRKO) model. Therefore, this conditional mouse model for PR ablation represents an invaluable resource with which to further define in a developmental and/or reproductive stage-specific manner the individual and integrative roles of distinct PR populations resident in multiple progesterone-responsive target sites.
(c) 2009 Wiley-Liss, Inc.
Figures



Similar articles
-
Generation of a mouse for conditional excision of progesterone receptor.Genesis. 2006 Aug;44(8):391-5. doi: 10.1002/dvg.20227. Genesis. 2006. PMID: 16868919
-
Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse.Mol Cell Biol. 2006 Sep;26(17):6571-83. doi: 10.1128/MCB.00654-06. Mol Cell Biol. 2006. PMID: 16914740 Free PMC article.
-
Cre-mediated recombination in cell lineages that express the progesterone receptor.Genesis. 2005 Feb;41(2):58-66. doi: 10.1002/gene.20098. Genesis. 2005. PMID: 15682389
-
Reproductive functions of progesterone receptors.Recent Prog Horm Res. 2002;57:339-55. doi: 10.1210/rp.57.1.339. Recent Prog Horm Res. 2002. PMID: 12017551 Review.
-
Steroid receptor coactivator 2 is essential for progesterone-dependent uterine function and mammary morphogenesis: insights from the mouse--implications for the human.J Steroid Biochem Mol Biol. 2006 Dec;102(1-5):22-31. doi: 10.1016/j.jsbmb.2006.09.007. Epub 2006 Oct 12. J Steroid Biochem Mol Biol. 2006. PMID: 17045797 Review.
Cited by
-
Enhancement of Female Rat Fertility via Ethanolic Extract from Nigella sativa L. (Black Cumin) Seeds Assessed via HPLC-ESI-MS/MS and Molecular Docking.Molecules. 2024 Feb 5;29(3):735. doi: 10.3390/molecules29030735. Molecules. 2024. PMID: 38338478 Free PMC article.
-
Impaired Progesterone-Responsiveness of CD11c+ Dendritic Cells Affects the Generation of CD4+ Regulatory T Cells and Is Associated With Intrauterine Growth Restriction in Mice.Front Endocrinol (Lausanne). 2019 Feb 25;10:96. doi: 10.3389/fendo.2019.00096. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30858825 Free PMC article.
-
Role of nuclear progesterone receptor isoforms in uterine pathophysiology.Hum Reprod Update. 2015 Mar-Apr;21(2):155-73. doi: 10.1093/humupd/dmu056. Epub 2014 Nov 18. Hum Reprod Update. 2015. PMID: 25406186 Free PMC article. Review.
-
Progesterone amplifies allergic inflammation and airway pathology in association with higher lung ILC2 responses.Am J Physiol Lung Cell Mol Physiol. 2024 Jul 1;327(1):L65-L78. doi: 10.1152/ajplung.00207.2023. Epub 2024 Apr 23. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38651968
-
Inactivation of the Progesterone Receptor in Mx1+ Cells Potentiates Osteogenesis in Calvaria but Not in Long Bone.PLoS One. 2015 Oct 2;10(10):e0139490. doi: 10.1371/journal.pone.0139490. eCollection 2015. PLoS One. 2015. PMID: 26431032 Free PMC article.
References
-
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19:1978–1990. - PubMed
-
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. - PubMed
-
- Hashimoto-Partyka MK, Lydon JP, Iruela-Arispe ML. Generation of a mouse for conditional excision of progesterone receptor. Genesis. 2006;44:391–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

