Synthesis and organization of hyaluronan and versican by embryonic stem cells undergoing embryoid body differentiation
- PMID: 20026669
- PMCID: PMC2842597
- DOI: 10.1369/jhc.2009.954826
Synthesis and organization of hyaluronan and versican by embryonic stem cells undergoing embryoid body differentiation
Abstract
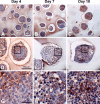
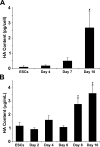
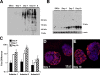
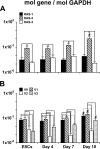
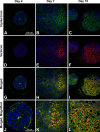
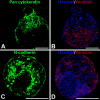
Embryonic stem cells (ESCs) provide a convenient model to probe the molecular and cellular dynamics of developmental cell morphogenesis. ESC differentiation in vitro via embryoid bodies (EBs) recapitulates many aspects of early stages of development, including the epithelial-mesenchymal transition (EMT) of pluripotent cells into more differentiated progeny. Hyaluronan and versican are important extracellular mediators of EMT processes, yet the temporal expression and spatial distribution of these extracellular matrix (ECM) molecules during EB differentiation remains undefined. Thus, the objective of this study was to evaluate the synthesis and organization of hyaluronan and versican by using murine ESCs during EB differentiation. Hyaluronan and versican (V0 and V1 isoforms), visualized by immunohistochemistry and evaluated biochemically, accumulated within EBs during the course of differentiation. Interestingly, increasing amounts of a 70-kDa proteolytic fragment of versican were also detected over time, along with ADAMTS-1 and -5 protein expression. ESCs expressed each of the hyaluronan synthases (HAS) -1, -2, and -3 and versican splice variants (V0, V1, V2, and V3) throughout EB differentiation, but HAS-2, V0, and V1 were expressed at significantly increased levels at each time point examined. Hyaluronan and versican exhibited overlapping expression patterns within EBs in regions of low cell density, and versican expression was excluded from clusters of epithelial (cytokeratin-positive) cells but was enriched within the vicinity of mesenchymal (N-cadherin-positive) cells. These results indicate that hyaluronan and versican synthesized by ESCs within EB microenvironments are associated with EMT processes and furthermore suggest that endogenously produced ECM molecules play a role in ESC differentiation. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials.
Figures

Similar articles
-
Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan.J Cell Biochem. 2010 Oct 15;111(3):585-96. doi: 10.1002/jcb.22744. J Cell Biochem. 2010. PMID: 20564236 Free PMC article.
-
Transforming growth factor-beta as a key molecule triggering the expression of versican isoforms v0 and v1, hyaluronan synthase-2 and synthesis of hyaluronan in malignant osteosarcoma cells.IUBMB Life. 2006 Jan;58(1):47-53. doi: 10.1080/15216540500531713. IUBMB Life. 2006. PMID: 16540432
-
Regulation of hyaluronan and versican deposition by growth factors in fibrosarcoma cell lines.Biochim Biophys Acta. 2008 Feb;1780(2):194-202. doi: 10.1016/j.bbagen.2007.10.005. Epub 2007 Oct 17. Biochim Biophys Acta. 2008. PMID: 17980161
-
Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation.Biotechnol Prog. 2009 Jan-Feb;25(1):43-51. doi: 10.1002/btpr.139. Biotechnol Prog. 2009. PMID: 19198003 Free PMC article. Review.
-
The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis.Matrix Biol. 2014 Apr;35:34-41. doi: 10.1016/j.matbio.2014.01.005. Epub 2014 Jan 18. Matrix Biol. 2014. PMID: 24444773 Free PMC article. Review.
Cited by
-
O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation.Dev Biol. 2010 Oct 1;346(1):25-38. doi: 10.1016/j.ydbio.2010.07.008. Epub 2010 Jul 14. Dev Biol. 2010. PMID: 20637190 Free PMC article.
-
Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential.Cell Tissue Res. 2012 Mar;347(3):701-11. doi: 10.1007/s00441-011-1215-5. Epub 2011 Aug 11. Cell Tissue Res. 2012. PMID: 21833761 Free PMC article.
-
Intrathecal delivery of mesenchymal stromal cells protects the structure of altered perineuronal nets in SOD1 rats and amends the course of ALS.Stem Cells. 2014 Dec;32(12):3163-72. doi: 10.1002/stem.1812. Stem Cells. 2014. PMID: 25113670 Free PMC article.
-
Hyaluronan regulates cell behavior: a potential niche matrix for stem cells.Biochem Res Int. 2012;2012:346972. doi: 10.1155/2012/346972. Epub 2012 Feb 12. Biochem Res Int. 2012. PMID: 22400115 Free PMC article.
-
Up-regulation of EMT-related gene VCAN by NPM1 mutant-driven TGF-β/cPML signalling promotes leukemia cell invasion.J Cancer. 2019 Oct 21;10(26):6570-6583. doi: 10.7150/jca.30223. eCollection 2019. J Cancer. 2019. PMID: 31777586 Free PMC article.
References
-
- Allison DD, Grande-Allen KJ (2006) Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng 12:2131–2140 - PubMed
-
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI (1995) Embryonic stem cells express neuronal properties in vitro. Dev Biol 168:342–357 - PubMed
-
- Behr R, Heneweer C, Viebahn C, Denker HW, Thie M (2005) Epithelial-mesenchymal transition in colonies of rhesus monkey embryonic stem cells: a model for processes involved in gastrulation. Stem Cells 23:805–816 - PubMed
-
- Carpenedo RL, Sargent CY, McDevitt TC (2007) Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells 25:2224–2234 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

