Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells
- PMID: 20026643
- PMCID: PMC2954245
- DOI: 10.1242/jcs.057976
Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells
Abstract
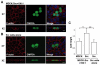
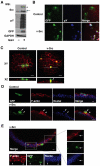
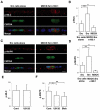
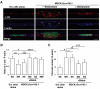
At the initial stage of carcinogenesis, transformation occurs in a single cell within an epithelial sheet. However, it remains unknown what happens at the boundary between normal and transformed cells. Using Madin-Darby canine kidney (MDCK) cells transformed with temperature-sensitive v-Src, we have examined the interface between normal and Src-transformed epithelial cells. We show that Src-transformed cells are apically extruded when surrounded by normal cells, but not when Src cells alone are cultured, suggesting that apical extrusion occurs in a cell-context-dependent manner. We also observe apical extrusion of Src-transformed cells in the enveloping layer of zebrafish gastrula embryos. When Src-transformed MDCK cells are surrounded by normal MDCK cells, myosin-II and focal adhesion kinase (FAK) are activated in Src cells, which further activate downstream mitogen-activated protein kinase (MAPK). Importantly, activation of these signalling pathways depends on the presence of surrounding normal cells and plays a crucial role in apical extrusion of Src cells. Collectively, these results indicate that interaction with surrounding normal epithelial cells influences the signalling pathways and behaviour of Src-transformed cells.
Figures



Similar articles
-
Accumulation of the myosin-II-spectrin complex plays a positive role in apical extrusion of Src-transformed epithelial cells.Genes Cells. 2018 Nov;23(11):974-981. doi: 10.1111/gtc.12643. Epub 2018 Oct 2. Genes Cells. 2018. PMID: 30175422
-
Characterization of the interface between normal and transformed epithelial cells.Nat Cell Biol. 2009 Apr;11(4):460-7. doi: 10.1038/ncb1853. Epub 2009 Mar 15. Nat Cell Biol. 2009. PMID: 19287376
-
EPLIN is a crucial regulator for extrusion of RasV12-transformed cells.J Cell Sci. 2015 Feb 15;128(4):781-9. doi: 10.1242/jcs.163113. Epub 2015 Jan 20. J Cell Sci. 2015. PMID: 25609711
-
Interface between normal and transformed epithelial cells: a road to a novel type of cancer prevention and treatment.Cancer Sci. 2011 Oct;102(10):1749-55. doi: 10.1111/j.1349-7006.2011.02011.x. Epub 2011 Jul 21. Cancer Sci. 2011. PMID: 21692919 Free PMC article. Review.
-
Impact of interactions between normal and transformed epithelial cells and the relevance to cancer.Cell Mol Life Sci. 2012 Jan;69(2):203-13. doi: 10.1007/s00018-011-0806-3. Epub 2011 Aug 30. Cell Mol Life Sci. 2012. PMID: 21877117 Free PMC article. Review.
Cited by
-
EPHA2-dependent outcompetition of KRASG12D mutant cells by wild-type neighbors in the adult pancreas.Curr Biol. 2021 Jun 21;31(12):2550-2560.e5. doi: 10.1016/j.cub.2021.03.094. Epub 2021 Apr 22. Curr Biol. 2021. PMID: 33891893 Free PMC article.
-
Generating suspended cell monolayers for mechanobiological studies.Nat Protoc. 2013 Dec;8(12):2516-30. doi: 10.1038/nprot.2013.151. Epub 2013 Nov 21. Nat Protoc. 2013. PMID: 24263091
-
Cell Extrusion: A Stress-Responsive Force for Good or Evil in Epithelial Homeostasis.Dev Cell. 2018 Feb 5;44(3):284-296. doi: 10.1016/j.devcel.2018.01.009. Epub 2018 Feb 5. Dev Cell. 2018. PMID: 29408235 Free PMC article. Review.
-
Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo.Nature. 2018 Feb 22;554(7693):523-527. doi: 10.1038/nature25742. Epub 2018 Feb 14. Nature. 2018. PMID: 29443958 Free PMC article.
-
Plectin is a novel regulator for apical extrusion of RasV12-transformed cells.Sci Rep. 2017 Mar 10;7:44328. doi: 10.1038/srep44328. Sci Rep. 2017. PMID: 28281696 Free PMC article.
References
-
- Abraham V., Chou M. L., DeBolt K. M., Koval M. (1999). Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am. J. Physiol. 276, L825-L834 - PubMed
-
- Baker N. E., Li W. (2008). Cell competition and its possible relation to cancer. Cancer Res. 68, 5505-5507 - PubMed
-
- Behrens J., Vakaet L., Friis R., Winterhager E., Van Roy F., Mareel M. M., Birchmeier W. (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120, 757-766 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

