Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana
- PMID: 20023197
- PMCID: PMC2814513
- DOI: 10.1105/tpc.109.070201
Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana
Abstract
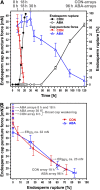
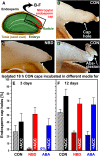
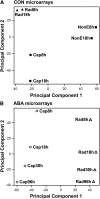
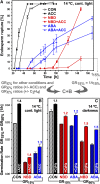
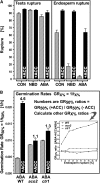
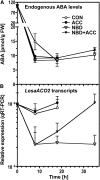
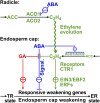
The micropylar endosperm cap covering the radicle in the mature seeds of most angiosperms acts as a constraint that regulates seed germination. Here, we report on a comparative seed biology study with the close Brassicaceae relatives Lepidium sativum and Arabidopsis thaliana showing that ethylene biosynthesis and signaling regulate seed germination by a mechanism that requires the coordinated action of the radicle and the endosperm cap. The larger seed size of Lepidium allows direct tissue-specific biomechanical, biochemical, and transcriptome analyses. We show that ethylene promotes endosperm cap weakening of Lepidium and endosperm rupture of both species and that it counteracts the inhibitory action of abscisic acid (ABA) on these two processes. Cross-species microarrays of the Lepidium micropylar endosperm cap and the radicle show that the ethylene-ABA antagonism involves both tissues and has the micropylar endosperm cap as a major target. Ethylene counteracts the ABA-induced inhibition without affecting seed ABA levels. The Arabidopsis loss-of-function mutants ACC oxidase2 (aco2; ethylene biosynthesis) and constitutive triple response1 (ethylene signaling) are impaired in the 1-aminocyclopropane-1-carboxylic acid (ACC)-mediated reversion of the ABA-induced inhibition of seed germination. Ethylene production by the ACC oxidase orthologs Lepidium ACO2 and Arabidopsis ACO2 appears to be a key regulatory step. Endosperm cap weakening and rupture are promoted by ethylene and inhibited by ABA to regulate germination in a process conserved across the Brassicaceae.
Figures


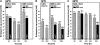
Similar articles
-
Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana.Plant Cell Physiol. 2006 Jul;47(7):864-77. doi: 10.1093/pcp/pcj059. Epub 2006 May 16. Plant Cell Physiol. 2006. PMID: 16705010
-
Regulation of seed germination in the close Arabidopsis relative Lepidium sativum: a global tissue-specific transcript analysis.Plant Physiol. 2011 Apr;155(4):1851-70. doi: 10.1104/pp.110.169706. Epub 2011 Feb 14. Plant Physiol. 2011. PMID: 21321254 Free PMC article.
-
Cross-species approaches to seed dormancy and germination: conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes.Plant Mol Biol. 2010 May;73(1-2):67-87. doi: 10.1007/s11103-009-9583-x. Epub 2009 Dec 15. Plant Mol Biol. 2010. PMID: 20013031
-
Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination.Plant Cell Rep. 2012 Feb;31(2):253-70. doi: 10.1007/s00299-011-1180-1. Epub 2011 Nov 2. Plant Cell Rep. 2012. PMID: 22044964 Review.
-
Underlying Biochemical and Molecular Mechanisms for Seed Germination.Int J Mol Sci. 2022 Jul 31;23(15):8502. doi: 10.3390/ijms23158502. Int J Mol Sci. 2022. PMID: 35955637 Free PMC article. Review.
Cited by
-
Ethylene, a Signaling Compound Involved in Seed Germination and Dormancy.Plants (Basel). 2024 Sep 24;13(19):2674. doi: 10.3390/plants13192674. Plants (Basel). 2024. PMID: 39409543 Free PMC article. Review.
-
Ethylene regulates post-germination seedling growth in wheat through spatial and temporal modulation of ABA/GA balance.J Exp Bot. 2020 Mar 25;71(6):1985-2004. doi: 10.1093/jxb/erz566. J Exp Bot. 2020. PMID: 31872216 Free PMC article.
-
Involvement of ethylene biosynthesis and perception during germination of dormant Avena fatua L. caryopses induced by KAR1 or GA3.Planta. 2019 Mar;249(3):719-738. doi: 10.1007/s00425-018-3032-5. Epub 2018 Oct 29. Planta. 2019. PMID: 30370496
-
Natural variation in germination responses of Arabidopsis to seasonal cues and their associated physiological mechanisms.Ann Bot. 2012 Jan;109(1):209-26. doi: 10.1093/aob/mcr264. Epub 2011 Oct 19. Ann Bot. 2012. PMID: 22012958 Free PMC article.
-
Identification of Quantitative Trait Loci Controlling Ethylene Production in Germinating Seeds in Maize (Zea mays L.).Sci Rep. 2020 Feb 3;10(1):1677. doi: 10.1038/s41598-020-58607-1. Sci Rep. 2020. PMID: 32015470 Free PMC article.
References
-
- Alonso, J.M., and Ecker, J.R. (2001). The ethylene pathway: A paradigm for plant hormone signaling and interaction. Sci. STKE 2001 RE1. - PubMed
-
- Bar-Or, C., Czosnek, H., and Koltai, H. (2007). Cross-species microarray hybridizations: A developing tool for studying species diversity. Trends Genet. 23 200–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

