Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer
- PMID: 20015196
- PMCID: PMC3823111
- DOI: 10.1111/j.1582-4934.2009.00994.x
Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer
Abstract
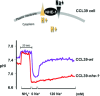
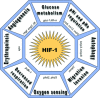
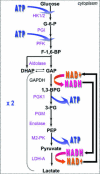
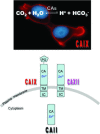
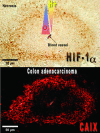
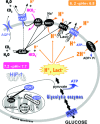
Maintenance of cellular pH homeostasis is fundamental to life. A number of key intracellular pH (pHi) regulating systems including the Na(+)/H(+) exchangers, the proton pump, the monocarboxylate transporters, the HCO(3)(-) transporters and exchangers and the membrane-associated and cytosolic carbonic anhydrases cooperate in maintaining a pHi that is permissive for cell survival. A common feature of tumours is acidosis caused by hypoxia (low oxygen tension). In addition to oncogene activation and transformation, hypoxia is responsible for inducing acidosis through a shift in cellular metabolism that generates a high acid load in the tumour microenvironment. However, hypoxia and oncogene activation also allow cells to adapt to the potentially toxic effects of an excess in acidosis. Hypoxia does so by inducing the activity of a transcription factor the hypoxia-inducible factor (HIF), and particularly HIF-1, that in turn enhances the expression of a number of pHi-regulating systems that cope with acidosis. In this review, we will focus on the characterization and function of some of the hypoxia-inducible pH-regulating systems and their induction by hypoxic stress. It is essential to understand the fundamentals of pH regulation to meet the challenge consisting in targeting tumour metabolism and acidosis as an anti-tumour approach. We will summarize strategies that take advantage of intracellular and extracellular pH regulation to target the primary tumour and metastatic growth, and to turn around resistance to chemotherapy and radiotherapy.
Figures


Similar articles
-
Hypoxia and cellular metabolism in tumour pathophysiology.J Physiol. 2017 Apr 15;595(8):2439-2450. doi: 10.1113/JP273309. Epub 2017 Feb 19. J Physiol. 2017. PMID: 28074546 Free PMC article. Review.
-
Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells.Oncotarget. 2017 Feb 7;8(6):10225-10237. doi: 10.18632/oncotarget.14379. Oncotarget. 2017. PMID: 28055960 Free PMC article.
-
Hypoxia and energetic tumour metabolism.Curr Opin Genet Dev. 2011 Feb;21(1):67-72. doi: 10.1016/j.gde.2010.10.006. Epub 2010 Nov 11. Curr Opin Genet Dev. 2011. PMID: 21074987 Review.
-
Targeting pH regulating proteins for cancer therapy-Progress and limitations.Semin Cancer Biol. 2017 Apr;43:66-73. doi: 10.1016/j.semcancer.2017.01.007. Epub 2017 Jan 27. Semin Cancer Biol. 2017. PMID: 28137473 Review.
-
Hypoxia and Its Acid-Base Consequences: From Mountains to Malignancy.Adv Exp Med Biol. 2016;903:301-23. doi: 10.1007/978-1-4899-7678-9_21. Adv Exp Med Biol. 2016. PMID: 27343105 Review.
Cited by
-
Synthesis and Biological Evaluation of Coumarin-Linked 4-Anilinomethyl-1,2,3-Triazoles as Potent Inhibitors of Carbonic Anhydrases IX and XIII Involved in Tumorigenesis.Metabolites. 2021 Apr 7;11(4):225. doi: 10.3390/metabo11040225. Metabolites. 2021. PMID: 33917033 Free PMC article.
-
Crystal structure of the Sema-PSI extracellular domain of human RON receptor tyrosine kinase.PLoS One. 2012;7(7):e41912. doi: 10.1371/journal.pone.0041912. Epub 2012 Jul 25. PLoS One. 2012. PMID: 22848655 Free PMC article.
-
Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs.Cancer Res. 2012 Jan 15;72(2):491-502. doi: 10.1158/0008-5472.CAN-11-2076. Epub 2011 Dec 1. Cancer Res. 2012. PMID: 22135092 Free PMC article.
-
The Role of the Hypoxia-Related Unfolded Protein Response (UPR) in the Tumor Microenvironment.Cancers (Basel). 2022 Oct 5;14(19):4870. doi: 10.3390/cancers14194870. Cancers (Basel). 2022. PMID: 36230792 Free PMC article. Review.
-
Tumour-specific metabolic adaptation to acidosis is coupled to epigenetic stability in osteosarcoma cells.Am J Cancer Res. 2016 Mar 15;6(4):859-75. eCollection 2016. Am J Cancer Res. 2016. PMID: 27186436 Free PMC article.
References
-
- Gillies RJ, Alger JR, Den Hollander JA, et al. Intracellular pH measured by NMR: methods and results. Kroc Found Ser. 1981;15:79–104. - PubMed
-
- Gillies RJ, Raghunand N, Garcia-Martin ML, et al. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 2004;23:57–64. - PubMed
-
- Gillies RJ, Liu Z, Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994;267:C195–203. - PubMed
-
- Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49:24S–42S. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

