The murine coronavirus nucleocapsid gene is a determinant of virulence
- PMID: 20007284
- PMCID: PMC2812375
- DOI: 10.1128/JVI.01758-09
The murine coronavirus nucleocapsid gene is a determinant of virulence
Abstract
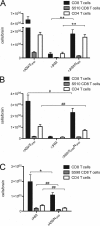
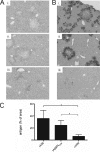
The murine coronavirus, mouse hepatitis virus (MHV) strain A59, causes acute encephalitis and chronic demyelinating disease as well as hepatitis in mice. The JHM strain (also called MHV-4 or JHM.SD) causes fatal encephalitis and only minimal hepatitis. Previous analysis of chimeric recombinant MHVs in which the spike gene, encoding the protein that mediates viral entry and cell-to-cell fusion, was exchanged between JHM and A59 showed that the spike plays a major role in determining organ tropism and neurovirulence but that other genes also play important roles in pathogenic outcome. Here, we have investigated the role of the nucleocapsid protein in MHV-induced disease. The multifunctional nucleocapsid protein is complexed with the genomic RNA, interacts with the viral membrane protein during virion assembly, and plays an import role in enhancing the efficiency of transcription. A pair of chimeric recombinant viruses in which the nucleocapsid gene was exchanged between JHM and A59 was selected and compared to wild-type parental strains in terms of virulence. Importantly, expression of the JHM nucleocapsid in the context of the A59 genome conferred increased mortality and spread of viral antigen in the mouse central nervous system compared to the parental A59 strain, while having little effect on the induction of hepatitis. While the JHM nucleocapsid did not appear to enhance neuron-to-neuron spread in primary neuronal cultures, the increased neurovirulence it conferred may be due in part to the induction of a less robust T-cell response than that induced by strain A59.
Figures



Similar articles
-
Murine coronavirus neuropathogenesis: determinants of virulence.J Neurovirol. 2010 Nov;16(6):427-34. doi: 10.3109/13550284.2010.529238. Epub 2010 Nov 12. J Neurovirol. 2010. PMID: 21073281 Free PMC article. Review.
-
Targeted recombination within the spike gene of murine coronavirus mouse hepatitis virus-A59: Q159 is a determinant of hepatotropism.J Virol. 1998 Dec;72(12):9628-36. doi: 10.1128/JVI.72.12.9628-9636.1998. J Virol. 1998. PMID: 9811696 Free PMC article.
-
Both spike and background genes contribute to murine coronavirus neurovirulence.J Virol. 2006 Jul;80(14):6834-43. doi: 10.1128/JVI.00432-06. J Virol. 2006. PMID: 16809289 Free PMC article.
-
Replicase genes of murine coronavirus strains A59 and JHM are interchangeable: differences in pathogenesis map to the 3' one-third of the genome.J Virol. 2007 Jan;81(2):1022-6. doi: 10.1128/JVI.01944-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079303 Free PMC article.
-
Pathogenesis of murine coronavirus in the central nervous system.J Neuroimmune Pharmacol. 2010 Sep;5(3):336-54. doi: 10.1007/s11481-010-9202-2. Epub 2010 Apr 6. J Neuroimmune Pharmacol. 2010. PMID: 20369302 Free PMC article. Review.
Cited by
-
Potential mouse models of coronavirus-related immune injury.Front Immunol. 2022 Sep 2;13:943783. doi: 10.3389/fimmu.2022.943783. eCollection 2022. Front Immunol. 2022. PMID: 36119040 Free PMC article. Review.
-
Genetic determinants of mouse hepatitis virus strain 1 pneumovirulence.J Virol. 2010 Sep;84(18):9278-91. doi: 10.1128/JVI.00330-10. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631137 Free PMC article.
-
Functional transcriptional regulatory sequence (TRS) RNA binding and helix destabilizing determinants of murine hepatitis virus (MHV) nucleocapsid (N) protein.J Biol Chem. 2012 Mar 2;287(10):7063-73. doi: 10.1074/jbc.M111.287763. Epub 2012 Jan 12. J Biol Chem. 2012. PMID: 22241479 Free PMC article.
-
Cell-type-specific type I interferon antagonism influences organ tropism of murine coronavirus.J Virol. 2011 Oct;85(19):10058-68. doi: 10.1128/JVI.05075-11. Epub 2011 Jul 13. J Virol. 2011. PMID: 21752905 Free PMC article.
-
Neurons cytoskeletal architecture remodeling during the replication cycle of mouse coronavirus MHV-JHM: a morphological in vitro study.BMC Vet Res. 2024 Jan 9;20(1):18. doi: 10.1186/s12917-023-03813-y. BMC Vet Res. 2024. PMID: 38195523 Free PMC article.
References
-
- Batts, K. P., and J. Ludwig. 1995. Chronic hepatitis. An update on terminology and reporting. Am. J. Surg. Pathol. 19:1409-1417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

