Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV
- PMID: 19995539
- PMCID: PMC2822069
- DOI: 10.1016/j.vaccine.2009.11.061
Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV
Abstract
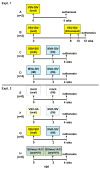
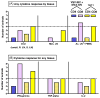
In a previously developed infant macaque model mimicking HIV infection by breast-feeding, we demonstrated that intramuscular immunization with recombinant poxvirus vaccines expressing simian immunodeficiency virus (SIV) structural proteins provided partial protection against infection following oral inoculation with virulent SIV. In an attempt to further increase systemic but also local antiviral immune responses at the site of viral entry, we tested the immunogenicity of different orally administered, replicating vaccines. One group of newborn macaques received an oral prime immunization with a recombinant vesicular stomatitis virus expressing SIVmac239 gag, pol and env (VSV-SIVgpe), followed 2 weeks later by an intramuscular boost immunization with MVA-SIV. Another group received two immunizations with live-attenuated SIVmac1A11, administered each time both orally and intravenously. Control animals received mock immunizations or non-SIV VSV and MVA control vectors. Analysis of SIV-specific immune responses in blood and lymphoid tissues at 4 weeks of age demonstrated that both vaccine regimens induced systemic antibody responses and both systemic and local cell-mediated immune responses. The safety and immunogenicity of the VSV-SIVgpe+MVA-SIV immunization regimen described in this report provide the scientific incentive to explore the efficacy of this vaccine regimen against virulent SIV exposure in the infant macaque model.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques.Vaccine. 2011 Apr 12;29(17):3124-37. doi: 10.1016/j.vaccine.2011.02.051. Epub 2011 Mar 4. Vaccine. 2011. PMID: 21377510 Free PMC article.
-
Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251.J Virol. 2003 Jan;77(1):179-90. doi: 10.1128/jvi.77.1.179-190.2003. J Virol. 2003. PMID: 12477823 Free PMC article.
-
Virus-Like Particles Displaying Trimeric Simian Immunodeficiency Virus (SIV) Envelope gp160 Enhance the Breadth of DNA/Modified Vaccinia Virus Ankara SIV Vaccine-Induced Antibody Responses in Rhesus Macaques.J Virol. 2016 Sep 12;90(19):8842-54. doi: 10.1128/JVI.01163-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466414 Free PMC article.
-
Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models.Expert Rev Vaccines. 2017 Oct;16(10):973-985. doi: 10.1080/14760584.2017.1371594. Epub 2017 Sep 4. Expert Rev Vaccines. 2017. PMID: 28838267 Free PMC article. Review.
-
Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment.Vaccine. 2016 Dec 12;34(51):6597-6609. doi: 10.1016/j.vaccine.2016.06.071. Epub 2016 Jul 6. Vaccine. 2016. PMID: 27395563 Free PMC article. Review.
Cited by
-
Vaccine-Elicited Mucosal and Systemic Antibody Responses Are Associated with Reduced Simian Immunodeficiency Viremia in Infant Rhesus Macaques.J Virol. 2016 Jul 27;90(16):7285-7302. doi: 10.1128/JVI.00481-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252535 Free PMC article.
-
HIV infection and specific mucosal immunity: workshop 4B.Adv Dent Res. 2011 Apr;23(1):142-51. doi: 10.1177/0022034511400222. Adv Dent Res. 2011. PMID: 21441496 Free PMC article.
-
The influence of delivery vectors on HIV vaccine efficacy.Front Microbiol. 2014 Aug 22;5:439. doi: 10.3389/fmicb.2014.00439. eCollection 2014. Front Microbiol. 2014. PMID: 25202303 Free PMC article. Review.
-
Replicon RNA Viral Vectors as Vaccines.Vaccines (Basel). 2016 Nov 7;4(4):39. doi: 10.3390/vaccines4040039. Vaccines (Basel). 2016. PMID: 27827980 Free PMC article. Review.
-
Potentiating cancer immunotherapy using an oncolytic virus.Mol Ther. 2010 Aug;18(8):1430-9. doi: 10.1038/mt.2010.98. Epub 2010 Jun 15. Mol Ther. 2010. PMID: 20551919 Free PMC article.
References
-
- Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362(9387):859–68. - PubMed
-
- The Breastfeeding and HIV International Transmission Study (BHITS) Group. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–66. - PubMed
-
- Shetty AK, Coovadia HM, Mirochnick MM, Maldonado Y, Mofenson LM, Eshleman SH, et al. Safety and trough concentrations of nevirapine prophylaxis given daily, twice weekly, or weekly in breast-feeding infants from birth to 6 months. J Acquired Immune Defic Syndr. 2003;34(5):482–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

