Proteomic analysis of post-translational modifications in conditioned Hermissenda
- PMID: 19961907
- PMCID: PMC2815081
- DOI: 10.1016/j.neuroscience.2009.11.066
Proteomic analysis of post-translational modifications in conditioned Hermissenda
Abstract
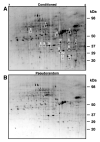
Post-translational modifications of proteins are a major determinant of biological function. Phosphorylation of proteins involved in signal transduction contributes to the induction and maintenance of several examples of cellular and synaptic plasticity. In this study we have identified phosphoproteins regulated by Pavlovian conditioning in lysates of Hermissenda nervous systems using two-dimensional electrophoresis (2DE) in conjunction with (32)P labeling, fluorescence based phosphoprotein in-gel staining, and mass spectrometry. Modification of protein phosphorylation regulated by conditioning was first assessed by densitometric analysis of (32)P labeled proteins resolved by 2DE from lysates of conditioned and pseudorandom control nervous systems. An independent assessment of phosphorylation regulated by conditioning was obtained from an examination of 2D gels stained with Pro-Q Diamond phosphoprotein dye. Mass spectrometric analysis of protein digests from phosphoprotein stained analytical gels or Coomassie Blue stained preparative gels provided for the identification of phosphoproteins that exhibited statistically significant increased phosphorylation in conditioned groups as compared to pseudorandom controls. A previously identified cytoskeletal related protein, Csp24 (24 kDa conditioned stimulus pathway phosphoprotein), involved in intermediate-term memory exhibited significantly increased phosphorylation detected 24 h post-conditioning. Our results show that proteins involved in diverse cellular functions such as transcriptional regulation, cell signaling, cytoskeletal regulation, metabolic activity, and protein degradation contribute to long-term post-translational modifications associated with Pavlovian conditioning.
Figures

Similar articles
-
Proteomic analysis of short- and intermediate-term memory in Hermissenda.Neuroscience. 2011 Sep 29;192:102-11. doi: 10.1016/j.neuroscience.2011.06.063. Epub 2011 Jun 28. Neuroscience. 2011. PMID: 21736919 Free PMC article.
-
Identification of a 24 kDa phosphoprotein associated with an intermediate stage of memory in Hermissenda.J Neurosci. 2000 May 15;20(10):RC74. doi: 10.1523/JNEUROSCI.20-10-j0002.2000. J Neurosci. 2000. PMID: 10783398 Free PMC article.
-
Inhibition of conditioned stimulus pathway phosphoprotein 24 expression blocks the development of intermediate-term memory in Hermissenda.J Neurosci. 2003 Apr 15;23(8):3415-22. doi: 10.1523/JNEUROSCI.23-08-03415.2003. J Neurosci. 2003. PMID: 12716949 Free PMC article.
-
Phosphoproteomics of human platelets: A quest for novel activation pathways.Biochim Biophys Acta. 2006 Dec;1764(12):1963-76. doi: 10.1016/j.bbapap.2006.08.017. Epub 2006 Sep 7. Biochim Biophys Acta. 2006. PMID: 17049321 Review.
-
A high-resolution two dimensional Gel- and Pro-Q DPS-based proteomics workflow for phosphoprotein identification and quantitative profiling.Methods Mol Biol. 2009;527:3-19, ix. doi: 10.1007/978-1-60327-834-8_1. Methods Mol Biol. 2009. PMID: 19241001 Review.
Cited by
-
Inhibition of Raf-MEK-ERK and hypoxia pathways by Phyllanthus prevents metastasis in human lung (A549) cancer cell line.BMC Complement Altern Med. 2013 Oct 20;13:271. doi: 10.1186/1472-6882-13-271. BMC Complement Altern Med. 2013. PMID: 24138815 Free PMC article.
-
Proteomic analysis of short- and intermediate-term memory in Hermissenda.Neuroscience. 2011 Sep 29;192:102-11. doi: 10.1016/j.neuroscience.2011.06.063. Epub 2011 Jun 28. Neuroscience. 2011. PMID: 21736919 Free PMC article.
References
-
- Ahn NG, Weiel JE, Chan CP, Krebs EG. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990;26:11487–11494. - PubMed
-
- Alkon DL, Nelson TJ. Specificity of molecular changes in neurons involved in memory storage. FASEB J. 1990;4:156–1576. - PubMed
-
- Ascoli G, Liu KX, Olds JL, Nelson TJ, Gusov PA, Bertucci C, Bramanti E, Raffaelli A, Salvador P, Alkon DL. Secondary structure of Ca2+-induced conformational change of calexcitin, a learning associated protein. J Biol Chem. 1997;272:29771–29779. - PubMed
-
- Baratier J, Peris L, Brocard J, Gory-Fauré S, Dufour F, Bose C, Fourest-Lieuvin A, Blanchoin L, Salin P, Job D, Andrieux A. Phosphorylation of microtubule-associated protein STOP by calmodulin kinase II. J Biol Chem. 2006;281:19561–19569. - PubMed
-
- Bouquet I, Boujemaa R, Cartier M-F, Préat T. Ciboulot regulates actin assembly during Drosophila brain metamorphosis. Cell. 2000;102:797–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

