Co-receptors and recognition of self at the immunological synapse
- PMID: 19960314
- PMCID: PMC5788015
- DOI: 10.1007/978-3-642-03858-7_9
Co-receptors and recognition of self at the immunological synapse
Abstract
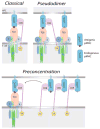
The co-receptors CD4 and CD8 are important in the activation of T cells primarily because of their ability to interact with the proteins of the MHC enhancing recognition of the MHC-peptide complex by the T cell receptor (TCR). An antigen-presenting cell presents a small number of antigenic peptides on its MHC molecules, in the presence of a much larger number of endogenous, mostly nonstimulatory, peptides. Recent work has demonstrated that these endogenous MHC-peptide complexes have an important role in modulating the sensitivity of the TCR. But the role of the endogenous nonstimulatory MHC-peptide complexes differs in MHC class I and class II-restricted T cells. This chapter discusses the data on the role of CD4 or CD8 co-receptors in T cell activation at the immunological synapse, and the role of non stimulatory MHC-peptide complexes in aiding antigen recognition.
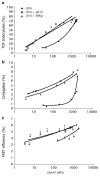
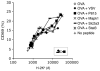
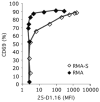
Figures
Similar articles
-
Distinct roles for CD4 and CD8 as co-receptors in antigen receptor signalling.Immunol Today. 1993 Apr;14(4):177-83. doi: 10.1016/0167-5699(93)90282-p. Immunol Today. 1993. PMID: 8499078 Review.
-
CD8 T cells, like CD4 T cells, are triggered by multivalent engagement of TCRs by MHC-peptide ligands but not by monovalent engagement.J Immunol. 2006 Feb 1;176(3):1498-505. doi: 10.4049/jimmunol.176.3.1498. J Immunol. 2006. PMID: 16424178
-
Opposite effects of endogenous peptide-MHC class I on T cell activity in the presence and absence of CD8.J Immunol. 2011 May 1;186(9):5193-200. doi: 10.4049/jimmunol.1003755. Epub 2011 Mar 30. J Immunol. 2011. PMID: 21451107 Free PMC article.
-
Quantitative contribution of CD4 and CD8 to T cell antigen receptor serial triggering.J Exp Med. 1997 Nov 17;186(10):1775-9. doi: 10.1084/jem.186.10.1775. J Exp Med. 1997. PMID: 9362538 Free PMC article.
-
Adaptive threshold-stochastic resonance (AT-SR) in MHC clusters on the cell surface.Immunol Lett. 2020 Jan;217:65-71. doi: 10.1016/j.imlet.2019.11.006. Epub 2019 Nov 15. Immunol Lett. 2020. PMID: 31738956 Review.
Cited by
-
Non-Stimulatory pMHC Enhance CD8 T Cell Effector Functions by Recruiting Coreceptor-Bound Lck.Front Immunol. 2021 Oct 11;12:721722. doi: 10.3389/fimmu.2021.721722. eCollection 2021. Front Immunol. 2021. PMID: 34707605 Free PMC article.
-
In Situ Detection of Salmonid Alphavirus 3 (SAV3) in Tissues of Atlantic Salmon in a Cohabitation Challenge Model with a Special Focus on the Immune Response to the Virus in the Pseudobranch.Viruses. 2023 Dec 17;15(12):2450. doi: 10.3390/v15122450. Viruses. 2023. PMID: 38140691 Free PMC article.
-
CD8αα and -αβ isotypes are equally recruited to the immunological synapse through their ability to bind to MHC class I.EMBO Rep. 2011 Dec 1;12(12):1251-6. doi: 10.1038/embor.2011.209. EMBO Rep. 2011. PMID: 22081144 Free PMC article.
-
Self-gratification yields not-so-naive T cells.Nat Immunol. 2014 Mar;15(3):217-9. doi: 10.1038/ni.2832. Nat Immunol. 2014. PMID: 24549065 No abstract available.
-
Structure, function, and immunomodulation of the CD8 co-receptor.Front Immunol. 2024 Aug 26;15:1412513. doi: 10.3389/fimmu.2024.1412513. eCollection 2024. Front Immunol. 2024. PMID: 39253084 Free PMC article. Review.
References
-
- Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NRJ. T cell receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. - PubMed
-
- Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NRJ, Travers PJ. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. - PubMed
-
- Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J Immunol. 2004;172:5887–5892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

