The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1
- PMID: 19933330
- PMCID: PMC2780317
- DOI: 10.1073/pnas.0911796106
The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1
Abstract
Both activated and resting CD4(+) T cells in mucosal tissues play important roles in the earliest phases of infection after sexual transmission of HIV-1, a process that is inefficient. HIV-1 gp120 binds to integrin alpha(4)beta(7) (alpha(4)beta(7)), the gut mucosal homing receptor. We find that alpha(4)beta(7)(high) CD4(+) T cells are more susceptible to productive infection than are alpha(4)beta(7)(low-neg) CD4(+) T cells in part because this cellular subset is enriched with metabolically active CD4(+) T cells. alpha(4)beta(7)(high) CD4(+) T cells are CCR5(high) and CXCR4(low); on these cells, alpha(4)beta(7) appears in a complex with CD4. The specific affinity of gp120 for alpha(4)beta(7) provides a mechanism for HIV-1 to target activated cells that are critical for efficient virus propagation and dissemination following sexual transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
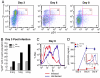

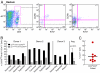
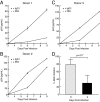
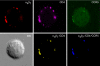
Similar articles
-
Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7.PLoS Pathog. 2012;8(5):e1002686. doi: 10.1371/journal.ppat.1002686. Epub 2012 May 31. PLoS Pathog. 2012. PMID: 22693444 Free PMC article.
-
Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility.J Immunol. 2011 Dec 1;187(11):6032-42. doi: 10.4049/jimmunol.1101836. Epub 2011 Nov 2. J Immunol. 2011. PMID: 22048765
-
HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV.J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. J Transl Med. 2011. PMID: 21284901 Free PMC article. Review.
-
Infection of CD127+ (interleukin-7 receptor+) CD4+ cells and overexpression of CTLA-4 are linked to loss of antigen-specific CD4 T cells during primary human immunodeficiency virus type 1 infection.J Virol. 2006 Oct;80(20):10162-72. doi: 10.1128/JVI.00249-06. J Virol. 2006. PMID: 17005693 Free PMC article.
-
Role of T-cell trafficking in the pathogenesis of HIV disease.Curr Opin HIV AIDS. 2019 Mar;14(2):115-120. doi: 10.1097/COH.0000000000000529. Curr Opin HIV AIDS. 2019. PMID: 30601238 Review.
Cited by
-
Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells.Cells. 2020 Sep 11;9(9):2076. doi: 10.3390/cells9092076. Cells. 2020. PMID: 32932813 Free PMC article.
-
HIV: cell binding and entry.Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a006866. doi: 10.1101/cshperspect.a006866. Cold Spring Harb Perspect Med. 2012. PMID: 22908191 Free PMC article. Review.
-
Comprehensive assessment of HIV target cells in the distal human gut suggests increasing HIV susceptibility toward the anus.J Acquir Immune Defic Syndr. 2013 Jul 1;63(3):263-71. doi: 10.1097/QAI.0b013e3182898392. J Acquir Immune Defic Syndr. 2013. PMID: 23392465 Free PMC article.
-
Immunodeficiency lentiviral infections in natural and non-natural hosts.Blood. 2011 Jul 28;118(4):847-54. doi: 10.1182/blood-2010-12-325936. Epub 2011 Apr 19. Blood. 2011. PMID: 21505193 Free PMC article. Review.
-
The Role of Integrin α4β7 in HIV Pathogenesis and Treatment.Curr HIV/AIDS Rep. 2018 Apr;15(2):127-135. doi: 10.1007/s11904-018-0382-3. Curr HIV/AIDS Rep. 2018. PMID: 29478152 Free PMC article. Review.
References
-
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. - PubMed
-
- Wagner N, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. - PubMed
-
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

