Cyclic AMP-mediated endocytosis of intestinal epithelial NHE3 requires binding to synaptotagmin 1
- PMID: 19926819
- PMCID: PMC2822502
- DOI: 10.1152/ajpgi.00379.2009
Cyclic AMP-mediated endocytosis of intestinal epithelial NHE3 requires binding to synaptotagmin 1
Abstract
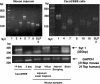
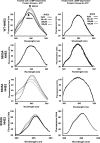
The apical membrane Na(+)-H(+) exchanger (NHE)3 is regulated by cAMP-dependent phosphorylation, which inhibits its activity through membrane endocytosis. The clathrin complex adaptor protein synaptotagmin 1 (Syt 1) appears to be essential to this process, but little is known about its expression in intestinal epithelial cells or interaction with NHE3. The intestinal epithelial expression and apical location of Syt 1 were determined by Syt 1 mRNA profiling and immunolocalization. Tandem mass spectrometry was used for protein identification. Bis(sulfosuccinimidyl) suberate (BS(3)) cross linking suggested that NHE3 and Syt 1 were in a membrane complex following cAMP stimulation of Caco2BBE (Brush Border Expressions) cells. To investigate the regulation of NHE3 appearance in a Syt 1-containing membrane compartment, doxycycline-inducible hemaglutinin (HA)-tagged NHE3 was expressed in Caco2BBE cells. HA-NHE3 correctly targeted to the apical membrane, where, upon cAMP stimulation, it was internalized with a Syt 1-containing compartment. Site-directed mutagenesis of NHE3 showed that serine 605 (S605) was pivotal to NHE3 and Syt 1 association and internalization. Direct Syt 1 interaction with NHE3 was suggested by fluorescence resonance energy transfer (FRET) analysis. The physiological role of S552 was less clear. By FRET, this serine residue appeared to be involved in cAMP-induced Syt 1 binding of NHE3. However, when HA-tagged NHE3 S552A was expressed in Caco2 cells, the mutated construct was not inserted into the apical membrane. We conclude that intestinal epithelial Syt 1 plays an important role in cAMP-stimulated endocytosis of apical NHE3 through cAMP-dependent phosphorylation of S605 that is required for NHE3 and Syt 1 association.
Figures



Similar articles
-
The recycling regulation of sodium-hydrogen exchanger isoform 3(NHE3) in epithelial cells.Cell Cycle. 2021 Dec;20(24):2565-2582. doi: 10.1080/15384101.2021.2005274. Epub 2021 Nov 25. Cell Cycle. 2021. PMID: 34822321 Free PMC article. Review.
-
Synaptotagmin I binds intestinal epithelial NHE3 and mediates cAMP- and Ca2+-induced endocytosis by recruitment of AP2 and clathrin.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1549-58. doi: 10.1152/ajpgi.00388.2006. Epub 2007 Feb 15. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17307723
-
Functional coupling of the downregulated in adenoma Cl-/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3.Am J Physiol Gastrointest Liver Physiol. 2009 Feb;296(2):G202-10. doi: 10.1152/ajpgi.90350.2008. Epub 2008 Dec 4. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19056765 Free PMC article.
-
Alpha2-adrenergic receptors attenuate secretagogue-induced endocytosis and promote exocytosis of intestinal NHE2 and NHE3.J Pharmacol Exp Ther. 2009 Sep;330(3):818-25. doi: 10.1124/jpet.109.151910. Epub 2009 Jun 25. J Pharmacol Exp Ther. 2009. PMID: 19556451 Free PMC article.
-
Short-term regulation of NHE3 by EGF and protein kinase C but not protein kinase A involves vesicle trafficking in epithelial cells and fibroblasts.Ann N Y Acad Sci. 2000;915:30-42. doi: 10.1111/j.1749-6632.2000.tb05221.x. Ann N Y Acad Sci. 2000. PMID: 11193592 Review.
Cited by
-
The recycling regulation of sodium-hydrogen exchanger isoform 3(NHE3) in epithelial cells.Cell Cycle. 2021 Dec;20(24):2565-2582. doi: 10.1080/15384101.2021.2005274. Epub 2021 Nov 25. Cell Cycle. 2021. PMID: 34822321 Free PMC article. Review.
-
Vasopressin Regulates Extracellular Vesicle Uptake by Kidney Collecting Duct Cells.J Am Soc Nephrol. 2016 Nov;27(11):3345-3355. doi: 10.1681/ASN.2015050568. Epub 2016 Mar 28. J Am Soc Nephrol. 2016. PMID: 27020854 Free PMC article.
-
Cell-specific effects of luminal acid, bicarbonate, cAMP, and carbachol on transporter trafficking in the intestine.Am J Physiol Gastrointest Liver Physiol. 2012 Oct 15;303(8):G937-50. doi: 10.1152/ajpgi.00452.2011. Epub 2012 Aug 30. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22936272 Free PMC article.
-
Regulation of electroneutral NaCl absorption by the small intestine.Annu Rev Physiol. 2011;73:261-81. doi: 10.1146/annurev-physiol-012110-142244. Annu Rev Physiol. 2011. PMID: 21054167 Free PMC article. Review.
-
Expression, localization, and functional role for synaptotagmins in pancreatic acinar cells.Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G306-16. doi: 10.1152/ajpgi.00108.2011. Epub 2011 Jun 2. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21636530 Free PMC article.
References
-
- Bryson A, Fletcher IS. Ligand exchange kinetics in the calcium-EDTA system. Aust J Chem 23: 1095–1110, 1970
-
- Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem 273: 32966–32972, 1998 - PubMed
-
- Chow CW, Khurana S, Woodside M, Grinstein S, Orlowski J. The epithelial Na(+)/H(+) exchanger, NHE3, is internalized through a clathrin-mediated pathway. J Biol Chem 274: 37551–37558, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

